Copy to clipboard
Copy to clipboard 
Joint replacement implants and techniques were priority topics for the largest orthopedic companies in attendance at the American Academy of Orthopaedic Surgeons Annual Meeting (AAOS). While enabling technologies like robotics and navigation dominated discussions, the top joint replacement companies emphasized that they remain focused on advancing implant portfolios and surgical techniques.
Zimmer Biomet, Stryker, DePuy Synthes and Smith+Nephew together account for 71.2% of global joint replacement sales. We project that the segment grew 6.5% and surpassed $21.5 billion in 2023. These companies expect a strong 2024 as procedure demand and adoption of new implants and enabling technology persist.
Here, we recap product launches and updates promoted at AAOS.
Zimmer Biomet Announces Shoulder Replacement Upgrades
In late 2022, Zimmer Biomet received FDA 510(k) clearance for the Identity Shoulder System for anatomic, reverse and revision shoulder replacement. The company said it will launch a stemless application of Identity to spare bone in active patients, as well as a suite of technology to help capitalize on the shoulder replacement market.
“Materials, technology and surgical techniques are getting better,” said Nnamdi Njoku, President of Sports Medicine, Surgical, Upper Extremities & Restorative Therapies at Zimmer Biomet. “Patients are becoming more aware that they don’t need to live with shoulder pain and are opting to undergo surgery sooner.”
Identity is a convertible system that uses proprietary technologies, such as Versa-Dial for infinite humeral head offset placement and Alliance Glenoid for a broad range of glenoid options. These technologies aim to align each surgeon’s approach to an individual patient’s anatomy.

Zimmer Biomet received FDA 510(k) clearance for the Identity Shoulder System for anatomic, reverse and revision shoulder replacement. It plans to launch a stemless version in the future.
Mr. Njoku noted that the use of enabling technology is key to the patient-specific approach used in shoulder replacement. Zimmer Biomet’s forthcoming robotic shoulder system was available at AAOS for controlled surgeon demos. The company plans to build off surgeon feedback on surgical workflow and positioning within the surgical field prior to the launch of the robot later this year.
Zimmer Biomet also showcased the soon-to-launch 2.0 version of the Signature ONE 3D-based surgical planning tool, which allows surgeons to tailor approaches to the unique anatomy of individual patients. The upgraded version will provide surgeons the ability to assess Identity’s humeral construct and conduct a 3D analysis of the joint’s range of motion to determine which implant would be the best fit for specific patients.
“Shoulder replacement volumes are growing by around 8%, and I don’t see that slowing down,” Mr. Njoku said. “I think enabling technology will spur a lot of that growth. Half of the ZB implants are planned in the Signature ONE platform, leading to personalized surgery that will help to grow shoulder replacement efficacy and interest in the procedure.”
Stryker Launches New Knee Revision System
Stryker introduced the Triathlon Hinge system, which is designed to reduce the procedural steps of a Triathlon revision-to-Hinge conversion during surgery. Triathlon Hinge allows the surgeon to keep the original tibial baseplate and offers simplified instrumentation, decreasing the number of trays needed for the procedure from 15 to three. The approach is versatile and streamlined, which leads to ease of use and cuts down on time in the O.R., said Lisa Kloes, Vice President and General Manager of Stryker’s Knees business.
“This system leverages the 20-year history of the Triathlon brand and design, but now offers versatility for the surgeon to move from a Triathlon TS to a Triathlon Hinge without changing the tibial baseplate,” Ms. Kloes said. “It’s a big deal to have that conversion capability. Surgeons only have to touch the femur, which is better for the patient and makes for a faster procedure. There are a lot of advantages to the system, and we’re the only company that offers that level of versatility.”

Triathlon Hinge allows the surgeon to keep the original tibial baseplate in a revision knee replacement.
Revisions account for 10% of Stryker’s $2.2 billion knee replacement business. Ms. Kloes said that even though revisions are a small portion of the company’s portfolio, it’s important to innovate in the space due to the complexity of the procedure.
In discussing trends in the broader joint replacement market, Ms. Kloes noted that technology will continue to evolve to make procedures less invasive and recovery times faster.
Stryker also touted its myMako app. The technology extends a surgeon’s Mako SmartRobotics experience to the app for the Apple Vision Pro and iPhone. When myMako is used on Apple Vision Pro, surgeons are able to visualize and review patients’ surgical plans.
“We aren’t only looking at the implant but how it is placed,” Ms. Kloes said. “Mako is all about putting in an implant as accurately and least invasively as possible to spare soft tissues. Knee replacement continues to evolve. There are opportunities on the implant side, as well as with digital and enabling technologies.”
Johnson & Johnson MedTech Takes Aim at Knee Replacement
Ethicon, a Johnson & Johnson MedTech company, promoted the publication of two studies that focused on best practice approaches to wound closure and dressing management in total knee and total hip replacement. The studies, which featured insight from 20 international orthopedic surgeons, were recently published in The Journal of Arthroplasty.
Surgical site infections, readmissions and revisions remain a concern for surgeons and companies. The study noted, however, that there’s little consensus from the literature and a lack of evidence generated in systematic reviews when it comes to wound closure.
The orthopedic surgeons who participated in the study identified the use of barbed over non-barbed sutures; the use of triclosan-coated sutures over non-antimicrobial-coated sutures; and the application of mesh adhesives over other skin closure methods as key interventions in joint replacement that are most focused on patient safety and improved outcomes.
“The biggest problem that we have in terms of revisions or morbidity following total knee and total hip arthroplasty is infection, and approximately half of the infections are attributable to wound healing problems,” said Michael A. Mont, M.D., Rubin Institute for Advanced Orthopedics and study co-author. “With the consensus reached by the participating orthopedic surgeons, we now have a guide that may help reduce the variability between surgeons and centers, promote standardization, and improve outcomes for patients after knee and hip replacements.”
DePuy Synthes, also of Johnson & Johnson MedTech, is focused on providing end-to-end procedural solutions for joint replacement surgery that focus on the value proposition of three key components: the implant, the surgical technique and the technology.
Sharrolyn Josse, Worldwide President of Joint Reconstruction at DePuy Synthes, highlighted the company’s efforts to optimize patient-specific alignment through the kinematic design of the ATTUNE Knee implant and the planning and precision of the VELYS enabling tech portfolio. “We’re considering how bone cuts, soft tissue balancing and surgical technique drive reproducibility and patient satisfaction,” she said.

VELYS recently surpassed 30,000 procedures, and the company noted its global expansion as a key growth driver for its knee replacement business.
ATTUNE, which is now in more than 2 million patients, is designed to match the natural kinematics of the knee. Joint replacement registries in the U.K. and U.S. show that use of the implant leads to quality outcomes and high patient satisfaction, according to Ms. Josse.
DePuy Synthes also showcased its VELYS Robotic-Assisted Solution, which seeks to optimize surgical workflows and help surgeons achieve reproducible outcomes. VELYS recently surpassed 30,000 procedures, and the company noted its global expansion as a key growth driver for its knee replacement business.
Smith+Nephew Launches New Total Shoulder System
Smith+Nephew commenced full U.S. commercial availability of its AETOS Shoulder System for anatomic and reverse shoulder replacement.
AETOS features the Meta Stem, which is designed for stability with metaphyseal fixation and an inlay collar, preservation of bone and maintenance of patient anatomy.
The company noted that the system requires fewer steps for conversion between anatomic and reverse techniques and fewer instruments for primary anatomic and reverse placement, which simplifies the O.R. workflow and provides value for procedures performed in an ASC setting.
“I’m thrilled with the stem fixation of the implant that AETOS provides and believe it will be a noticeable, positive improvement for my patients,” said Matthew Ramsey, M.D., Shoulder and Elbow Specialist at Rothman Orthopaedics. “I’m also very pleased with the instrumentation, especially the glenoid reamer, being so low profile and easy to use.”
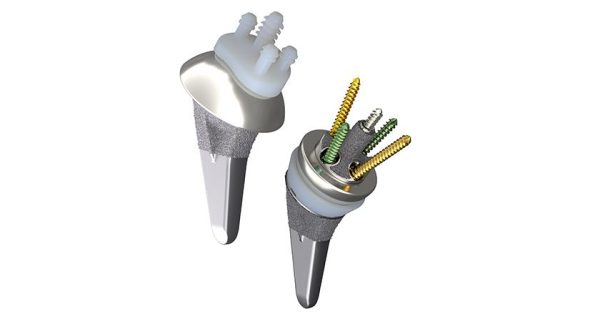
The newly launched AETOS features the Meta Stem, which is designed for stability with metaphyseal fixation and an inlay collar, preservation of bone and maintenance of patient anatomy.
Smith+Nephew also announced FDA 510(k) clearance for AETOS’ use with the ATLASPLAN 3D Planning Software and Patient Specific Instrumentation for total shoulder replacement. ATLASPLAN was developed in partnership with Materialise. It can be accessed from any computer or tablet, offers fast turnaround time from image upload to planning and provides an optional 3D-printed glenoid guide to provide a reliable and accurate method to execute the surgical plan.
Shoulder replacement accounts for less than 2% of Smith+Nephew’s total joint replacement revenue, which we project will surpass $1.6 billion in 2023. Smith+Nephew CFO Anne-Francoise Nesmes recently said the launch of AETOS will make the company “competitive for the first time in that large and high-growth category.”
Joint replacement implants and techniques were priority topics for the largest orthopedic companies in attendance at the American Academy of Orthopaedic Surgeons Annual Meeting (AAOS). While enabling technologies like robotics and navigation dominated discussions, the top joint replacement companies emphasized that they remain focused on...
Joint replacement implants and techniques were priority topics for the largest orthopedic companies in attendance at the American Academy of Orthopaedic Surgeons Annual Meeting (AAOS). While enabling technologies like robotics and navigation dominated discussions, the top joint replacement companies emphasized that they remain focused on advancing implant portfolios and surgical techniques.
Zimmer Biomet, Stryker, DePuy Synthes and Smith+Nephew together account for 71.2% of global joint replacement sales. We project that the segment grew 6.5% and surpassed $21.5 billion in 2023. These companies expect a strong 2024 as procedure demand and adoption of new implants and enabling technology persist.
Here, we recap product launches and updates promoted at AAOS.
Zimmer Biomet Announces Shoulder Replacement Upgrades
In late 2022, Zimmer Biomet received FDA 510(k) clearance for the Identity Shoulder System for anatomic, reverse and revision shoulder replacement. The company said it will launch a stemless application of Identity to spare bone in active patients, as well as a suite of technology to help capitalize on the shoulder replacement market.
“Materials, technology and surgical techniques are getting better,” said Nnamdi Njoku, President of Sports Medicine, Surgical, Upper Extremities & Restorative Therapies at Zimmer Biomet. “Patients are becoming more aware that they don’t need to live with shoulder pain and are opting to undergo surgery sooner.”
Identity is a convertible system that uses proprietary technologies, such as Versa-Dial for infinite humeral head offset placement and Alliance Glenoid for a broad range of glenoid options. These technologies aim to align each surgeon’s approach to an individual patient’s anatomy.

Zimmer Biomet received FDA 510(k) clearance for the Identity Shoulder System for anatomic, reverse and revision shoulder replacement. It plans to launch a stemless version in the future.
Mr. Njoku noted that the use of enabling technology is key to the patient-specific approach used in shoulder replacement. Zimmer Biomet’s forthcoming robotic shoulder system was available at AAOS for controlled surgeon demos. The company plans to build off surgeon feedback on surgical workflow and positioning within the surgical field prior to the launch of the robot later this year.
Zimmer Biomet also showcased the soon-to-launch 2.0 version of the Signature ONE 3D-based surgical planning tool, which allows surgeons to tailor approaches to the unique anatomy of individual patients. The upgraded version will provide surgeons the ability to assess Identity’s humeral construct and conduct a 3D analysis of the joint’s range of motion to determine which implant would be the best fit for specific patients.
“Shoulder replacement volumes are growing by around 8%, and I don’t see that slowing down,” Mr. Njoku said. “I think enabling technology will spur a lot of that growth. Half of the ZB implants are planned in the Signature ONE platform, leading to personalized surgery that will help to grow shoulder replacement efficacy and interest in the procedure.”
Stryker Launches New Knee Revision System
Stryker introduced the Triathlon Hinge system, which is designed to reduce the procedural steps of a Triathlon revision-to-Hinge conversion during surgery. Triathlon Hinge allows the surgeon to keep the original tibial baseplate and offers simplified instrumentation, decreasing the number of trays needed for the procedure from 15 to three. The approach is versatile and streamlined, which leads to ease of use and cuts down on time in the O.R., said Lisa Kloes, Vice President and General Manager of Stryker’s Knees business.
“This system leverages the 20-year history of the Triathlon brand and design, but now offers versatility for the surgeon to move from a Triathlon TS to a Triathlon Hinge without changing the tibial baseplate,” Ms. Kloes said. “It’s a big deal to have that conversion capability. Surgeons only have to touch the femur, which is better for the patient and makes for a faster procedure. There are a lot of advantages to the system, and we’re the only company that offers that level of versatility.”

Triathlon Hinge allows the surgeon to keep the original tibial baseplate in a revision knee replacement.
Revisions account for 10% of Stryker’s $2.2 billion knee replacement business. Ms. Kloes said that even though revisions are a small portion of the company’s portfolio, it’s important to innovate in the space due to the complexity of the procedure.
In discussing trends in the broader joint replacement market, Ms. Kloes noted that technology will continue to evolve to make procedures less invasive and recovery times faster.
Stryker also touted its myMako app. The technology extends a surgeon’s Mako SmartRobotics experience to the app for the Apple Vision Pro and iPhone. When myMako is used on Apple Vision Pro, surgeons are able to visualize and review patients’ surgical plans.
“We aren’t only looking at the implant but how it is placed,” Ms. Kloes said. “Mako is all about putting in an implant as accurately and least invasively as possible to spare soft tissues. Knee replacement continues to evolve. There are opportunities on the implant side, as well as with digital and enabling technologies.”
Johnson & Johnson MedTech Takes Aim at Knee Replacement
Ethicon, a Johnson & Johnson MedTech company, promoted the publication of two studies that focused on best practice approaches to wound closure and dressing management in total knee and total hip replacement. The studies, which featured insight from 20 international orthopedic surgeons, were recently published in The Journal of Arthroplasty.
Surgical site infections, readmissions and revisions remain a concern for surgeons and companies. The study noted, however, that there’s little consensus from the literature and a lack of evidence generated in systematic reviews when it comes to wound closure.
The orthopedic surgeons who participated in the study identified the use of barbed over non-barbed sutures; the use of triclosan-coated sutures over non-antimicrobial-coated sutures; and the application of mesh adhesives over other skin closure methods as key interventions in joint replacement that are most focused on patient safety and improved outcomes.
“The biggest problem that we have in terms of revisions or morbidity following total knee and total hip arthroplasty is infection, and approximately half of the infections are attributable to wound healing problems,” said Michael A. Mont, M.D., Rubin Institute for Advanced Orthopedics and study co-author. “With the consensus reached by the participating orthopedic surgeons, we now have a guide that may help reduce the variability between surgeons and centers, promote standardization, and improve outcomes for patients after knee and hip replacements.”
DePuy Synthes, also of Johnson & Johnson MedTech, is focused on providing end-to-end procedural solutions for joint replacement surgery that focus on the value proposition of three key components: the implant, the surgical technique and the technology.
Sharrolyn Josse, Worldwide President of Joint Reconstruction at DePuy Synthes, highlighted the company’s efforts to optimize patient-specific alignment through the kinematic design of the ATTUNE Knee implant and the planning and precision of the VELYS enabling tech portfolio. “We’re considering how bone cuts, soft tissue balancing and surgical technique drive reproducibility and patient satisfaction,” she said.

VELYS recently surpassed 30,000 procedures, and the company noted its global expansion as a key growth driver for its knee replacement business.
ATTUNE, which is now in more than 2 million patients, is designed to match the natural kinematics of the knee. Joint replacement registries in the U.K. and U.S. show that use of the implant leads to quality outcomes and high patient satisfaction, according to Ms. Josse.
DePuy Synthes also showcased its VELYS Robotic-Assisted Solution, which seeks to optimize surgical workflows and help surgeons achieve reproducible outcomes. VELYS recently surpassed 30,000 procedures, and the company noted its global expansion as a key growth driver for its knee replacement business.
Smith+Nephew Launches New Total Shoulder System
Smith+Nephew commenced full U.S. commercial availability of its AETOS Shoulder System for anatomic and reverse shoulder replacement.
AETOS features the Meta Stem, which is designed for stability with metaphyseal fixation and an inlay collar, preservation of bone and maintenance of patient anatomy.
The company noted that the system requires fewer steps for conversion between anatomic and reverse techniques and fewer instruments for primary anatomic and reverse placement, which simplifies the O.R. workflow and provides value for procedures performed in an ASC setting.
“I’m thrilled with the stem fixation of the implant that AETOS provides and believe it will be a noticeable, positive improvement for my patients,” said Matthew Ramsey, M.D., Shoulder and Elbow Specialist at Rothman Orthopaedics. “I’m also very pleased with the instrumentation, especially the glenoid reamer, being so low profile and easy to use.”

The newly launched AETOS features the Meta Stem, which is designed for stability with metaphyseal fixation and an inlay collar, preservation of bone and maintenance of patient anatomy.
Smith+Nephew also announced FDA 510(k) clearance for AETOS’ use with the ATLASPLAN 3D Planning Software and Patient Specific Instrumentation for total shoulder replacement. ATLASPLAN was developed in partnership with Materialise. It can be accessed from any computer or tablet, offers fast turnaround time from image upload to planning and provides an optional 3D-printed glenoid guide to provide a reliable and accurate method to execute the surgical plan.
Shoulder replacement accounts for less than 2% of Smith+Nephew’s total joint replacement revenue, which we project will surpass $1.6 billion in 2023. Smith+Nephew CFO Anne-Francoise Nesmes recently said the launch of AETOS will make the company “competitive for the first time in that large and high-growth category.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
CL
Carolyn LaWell is ORTHOWORLD's Chief Content Officer. She joined ORTHOWORLD in 2012 to oversee its editorial and industry education. She previously served in editor roles at B2B magazines and newspapers.