
 Copy to clipboard
Copy to clipboard 
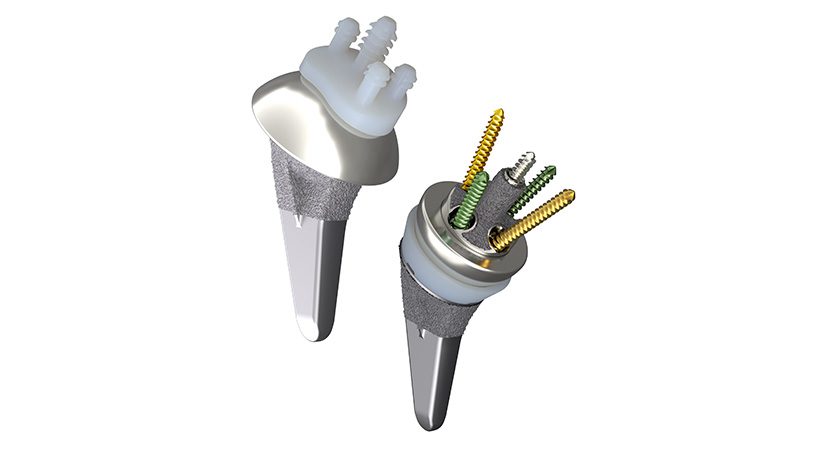
Smith+Nephew commenced full commercial availability of its AETOS Shoulder System in the U.S., along with FDA 510(k) clearance for its use with ATLASPLAN 3D Planning Software and Patient Specific Instrumentation for total shoulder arthroplasty.
Developed to restore patients’ range-of-motion and help minimize arthritic shoulder pain, the AETOS Shoulder System features the Meta Stem which is designed for stability with metaphyseal fixation and an inlay collar, bone preservation and to maintain patient anatomy.
Indicated for both anatomic and reverse total shoulder arthroplasty, the AETOS Shoulder offers a compact yet comprehensive portfolio of solutions designed to enhance the surgical experience by enabling intraoperative flexibility. With fewer steps for conversion and fewer instruments for primary anatomic and reverse, the system is designed to simplify the operating room flow. This also allows for an efficient solution, particularly of value in an Ambulatory Service Center.
ATLASPLAN 3D Planning Software and Patient Specific Instrumentation enables pre-operative case planning for total shoulder arthroplasty, and was developed in partnership with Materialise. It features:
- A user-friendly online web-based planner for access from any computer or tablet.
- Fast turnaround time from image upload to planning.
- An optional 3D-printed glenoid guide, designed with the patented coracoid clip for stability, provides a reliable and accurate method to execute the surgical plan through conventional surgical techniques.
AETOS received FDA 510(k) marketing clearance in 2023.
Source: Smith+Nephew
Smith+Nephew commenced full commercial availability of its AETOS Shoulder System in the U.S., along with FDA 510(k) clearance for its use with ATLASPLAN 3D Planning Software and Patient Specific Instrumentation for total shoulder arthroplasty.
Developed to restore patients’ range-of-motion and help minimize arthritic shoulder pain, the AETOS...
Smith+Nephew commenced full commercial availability of its AETOS Shoulder System in the U.S., along with FDA 510(k) clearance for its use with ATLASPLAN 3D Planning Software and Patient Specific Instrumentation for total shoulder arthroplasty.
Developed to restore patients’ range-of-motion and help minimize arthritic shoulder pain, the AETOS Shoulder System features the Meta Stem which is designed for stability with metaphyseal fixation and an inlay collar, bone preservation and to maintain patient anatomy.
Indicated for both anatomic and reverse total shoulder arthroplasty, the AETOS Shoulder offers a compact yet comprehensive portfolio of solutions designed to enhance the surgical experience by enabling intraoperative flexibility. With fewer steps for conversion and fewer instruments for primary anatomic and reverse, the system is designed to simplify the operating room flow. This also allows for an efficient solution, particularly of value in an Ambulatory Service Center.
ATLASPLAN 3D Planning Software and Patient Specific Instrumentation enables pre-operative case planning for total shoulder arthroplasty, and was developed in partnership with Materialise. It features:
- A user-friendly online web-based planner for access from any computer or tablet.
- Fast turnaround time from image upload to planning.
- An optional 3D-printed glenoid guide, designed with the patented coracoid clip for stability, provides a reliable and accurate method to execute the surgical plan through conventional surgical techniques.
AETOS received FDA 510(k) marketing clearance in 2023.
Source: Smith+Nephew

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







