Hip replacement has evolved significantly since the earliest attempts. The latest product launches focus on navigation and resurfacing.
July 9, 2025
,
Julie A. Vetalice
July 8, 2025
,
Julie A. Vetalice
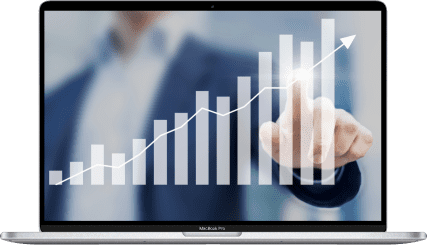
The Numbers
Our chart of the month tracks Globus Medical’s quarterly pro forma growth since the beginning of 2023, showing its overall solid performance.
The company’s aprevo Technology Platform combines proprietary AI-driven software with patient-specific fusion devices to personalize surgical procedures, improve patient outcomes and reduce complications.
Orthoflash®
The system supports treatment of scapular fractures using several different techniques, including single and dual plating.
July 9, 2025
,
Julie A. Vetalice
VISIE’s scanning platform is completely non-invasive and employs no bone-pinned trackers, clamps or registration steps.
July 9, 2025
,
Julie A. Vetalice
The novel device is designed to provide a minimally invasive option for patients suffering from facet joint-related pain or instability.
July 9, 2025
,
Julie A. Vetalice
Highridge licensed U.S. rights to activL and is commencing production for the launch of its own activL lumbar disc later this year.
July 8, 2025
,
Julie A. Vetalice
Of the more than 350 spinal bone grafts in the U.S. market, Cerapedics has two of only three spinal bone grafts that have successfully gone through the PMA process.
July 8, 2025
,
Julie A. Vetalice
Competitive Landscape
Industry Voices