
 Copy to clipboard
Copy to clipboard 
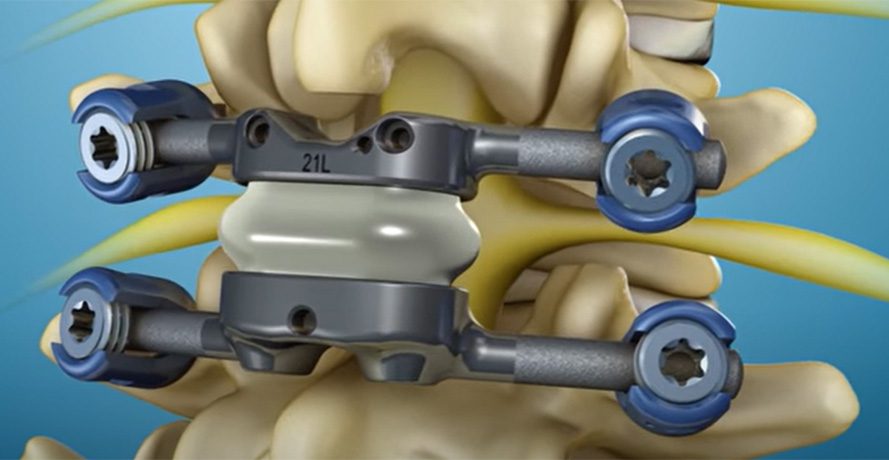
Ron Sacher recognized a need for a better solution to treat patients with spinal stenosis and degenerative spondylolisthesis. He thought this targeted patient population would benefit from stabilizing the lumbar spine and re-establishing controlled motion instead of fusing their vertebrae. Twenty years in the making, Premia Spine has received FDA Premarket Approval for its TOPS System.
The facet joint replacement system is a differentiated technology in the spine market’s motion preservation space. The system replaces the soft and bony tissue removed during decompression surgery and is designed to be used between segments L2 and L5.
Premia Spine originally received its CE Mark and has worked at sites in Europe to collect data and fine-tune the product and technique. The company’s U.S. plans involve a limited launch in 2023 and a full launch in 2024.
“We want to make sure that patient number 10 does as well as patient number one,” said Mr. Sacher, Founder and CEO of Premia Spine. “We have to make sure that the foundation is built strong and soundly. We’re in no hurry to move fast. We want to be successful and laser focused.”
We spoke with Mr. Sacher and Pete Wehrly, President of Premia Spine U.S., to discuss the technology’s genesis, commercialization plans and the company’s future.
Why is this technology a must for the spine market?
Mr. Sacher: We were initially involved in the hip and knee business, and had access to a unique material that replicated the connection of cartilage. We realized that it would take 15 years of clinical data to prove that our alternative to the gold standard was worth the effort. The bar was so high because the results in traditional large joints are so good. We concluded that our time and resources were better spent looking for an application that was underserving a patient population.
As soon as we looked at spine, we realized that results are not nearly as good as they are in large joints. We concluded that the solutions aren’t physiological, and maybe that’s why the clinical outcomes in spine are not nearly as good as in large joints.
At that time, there was a lot of hype around lumbar disc replacement. We decided not to pursue it because it was well-researched and served. Conversely, in the posterior spine, little technology made sense to us. The technologies were mainly four pedicle screws connected with a flexible rod or spring that looked like fusion, but didn’t behave like the natural spine.
We knew a joint was there, and it was the culprit for stenosis and spondy. We thought, why don’t we remove that joint, put in an artificial joint that replicates the function of the facet joint and see if we can get better outcomes? As soon as we started clinical work with the original TOPS device, we saw the benefits associated with facet replacement.
You published an update on your IDE study earlier this year. What were some of the results?
Mr. Sacher: TOPS outperformed fusion in every category we measured: fewer revisions; less opioid use; improved ODI, which measures function; leg pain reduction; back pain reduction.
It’s a testament to two things. First, good patient selection. We need to understand your pathology and ensure that it matches what TOPS can do. Fortunately, it’s very easy to spot these patients clinically and radiographically.
The second key is to remove all the offending elements that are pressing on the nerve roots. If you do a good decompression, patients can get better, and that’s where there’s a paradigm shift with our technology. Fusion procedures require cutting the bone to create room for the nerves to move their impingement, but you still have to leave enough bone to get through that bony bridge. If you do a wide decompression, it’s hard for the bridge to take hold.
We come in and tell the doctor look, you can perform as wide a decompression as you want. We don’t need a bony bridge. Make sure there’s no nerve root impingement — not today, not tomorrow. Put in our device and replicate the normal kinematics and physiology of the spine.
We knew going into the study that we would get good results. It came down to how well we executed. Jeff Withrow, our V.P. of Clinical and Reimbursement, ran a great study. He picked great sites and did great training. Surgeons chose the right patients. That reflects the potential of this technology when it’s used properly.
What does your rollout plan look like, once you receive approval?
Mr. Wehrly: It will be very focused. We have 37 sites plus another 10 physicians who went through cadaveric training. We’ll start with those institutions. Now that surgeons have seen the data, they’re calling patients. They have bought into the solution. In many of the institutions we’re working with, there are more doctors that we need to train and onboard. We’ll have three sales managers in the U.S. to find incremental distribution beyond those first 37.
I think the physicians in the know and coming out of residency or fellowship understand the benefits of motion and will be open to speaking with us. And at the end of the day, there’s no question that data is king.
The industry has been talking about motion preservation for decades. Several companies are seeking to differentiate in the space. What will the industry be saying about motion preservation in five years?
Mr. Sacher: There was a lot of excitement when motion preservation emerged in the early 2000s. My understanding has matured consistently with how the industry looks at motion preservation. Initially, I was naive and thought that anything that moves must be good. Then we started to see that’s not true. You can have products that move that are not designed to withstand the forces of the spine or sync with the spine. People went from initial excitement to realizing that it would not solve every problem, and it was overused or abused.
At some point, surgeons stepped back and said, we’re not sure if motion preservation makes sense, and it almost disappeared. It disappeared from the lumbar spine. But there was enough of an ember that when the flames were fanned, it managed to catch. The reason it caught was some really good companies had really good products. They did good research to show that in cervical disc replacement, you have a product that’s at least as good as fusion, if not better, when you look at two levels. Those companies learned from other companies’ mistakes and tried to maintain being on label and focusing on the right patient population. That evolution began what we’re seeing in the upswing of motion preservation.
I think we’re starting to see lumbar disc replacement also gather strength.
Five years from now, most surgeons will perform cervical disc replacements as their go-to procedure. Lumbar disc replacement will be a product of choice for the right patient. TOPS will be the gold standard for patients with stenosis and spondy.
What can we expect from Premia Spine in the next five years?
Mr. Sacher: The next big focus for us is the issue of reimbursement at the center level and the surgeon level.
Reimbursement will be great for the facilities because we’ll probably get the new technology add-on payment. Now we’re focused on surgeon payment. We want to make sure that we take care of the doctors, and they get paid what they deserve to get paid for this type of procedure.
We have more products in our pipeline that we’re developing, and we’ll get through clinicals to get those approved to expand indications for TOPS.
We’re going to stick with PMAs. We have another platform that we’re super excited about that we’ll start working on as well. It’s completely different from TOPS and has a huge market application.
What are some of the lessons that you’ve learned just from the PMA process?
Mr. Sacher: First, I highly recommend filing for a Breakthrough Device Designation if you can. Second, engage FDA in the interactive process. We did, and I think it was extremely helpful. Instead of just filing for a PMA, we broke our PMA into three modules. We worked with FDA through each of the three subsequent modules, starting with in vitro testing and biocompatibility, moving on to manufacturing and quality, and then finally moving into the clinical piece. That was a very good way of working with the agency. Engage the agency early and work with them closely. Those are the two big takeaways.
Also, make sure you raise enough money to finance the technology. PMAs are extremely expensive.
Ron Sacher recognized a need for a better solution to treat patients with spinal stenosis and degenerative spondylolisthesis. He thought this targeted patient population would benefit from stabilizing the lumbar spine and re-establishing controlled motion instead of fusing their vertebrae. Twenty years in the making, Premia Spine has received...
Ron Sacher recognized a need for a better solution to treat patients with spinal stenosis and degenerative spondylolisthesis. He thought this targeted patient population would benefit from stabilizing the lumbar spine and re-establishing controlled motion instead of fusing their vertebrae. Twenty years in the making, Premia Spine has received FDA Premarket Approval for its TOPS System.
The facet joint replacement system is a differentiated technology in the spine market’s motion preservation space. The system replaces the soft and bony tissue removed during decompression surgery and is designed to be used between segments L2 and L5.
Premia Spine originally received its CE Mark and has worked at sites in Europe to collect data and fine-tune the product and technique. The company’s U.S. plans involve a limited launch in 2023 and a full launch in 2024.
“We want to make sure that patient number 10 does as well as patient number one,” said Mr. Sacher, Founder and CEO of Premia Spine. “We have to make sure that the foundation is built strong and soundly. We’re in no hurry to move fast. We want to be successful and laser focused.”
We spoke with Mr. Sacher and Pete Wehrly, President of Premia Spine U.S., to discuss the technology’s genesis, commercialization plans and the company’s future.
Why is this technology a must for the spine market?
Mr. Sacher: We were initially involved in the hip and knee business, and had access to a unique material that replicated the connection of cartilage. We realized that it would take 15 years of clinical data to prove that our alternative to the gold standard was worth the effort. The bar was so high because the results in traditional large joints are so good. We concluded that our time and resources were better spent looking for an application that was underserving a patient population.
As soon as we looked at spine, we realized that results are not nearly as good as they are in large joints. We concluded that the solutions aren’t physiological, and maybe that’s why the clinical outcomes in spine are not nearly as good as in large joints.
At that time, there was a lot of hype around lumbar disc replacement. We decided not to pursue it because it was well-researched and served. Conversely, in the posterior spine, little technology made sense to us. The technologies were mainly four pedicle screws connected with a flexible rod or spring that looked like fusion, but didn’t behave like the natural spine.
We knew a joint was there, and it was the culprit for stenosis and spondy. We thought, why don’t we remove that joint, put in an artificial joint that replicates the function of the facet joint and see if we can get better outcomes? As soon as we started clinical work with the original TOPS device, we saw the benefits associated with facet replacement.
You published an update on your IDE study earlier this year. What were some of the results?
Mr. Sacher: TOPS outperformed fusion in every category we measured: fewer revisions; less opioid use; improved ODI, which measures function; leg pain reduction; back pain reduction.
It’s a testament to two things. First, good patient selection. We need to understand your pathology and ensure that it matches what TOPS can do. Fortunately, it’s very easy to spot these patients clinically and radiographically.
The second key is to remove all the offending elements that are pressing on the nerve roots. If you do a good decompression, patients can get better, and that’s where there’s a paradigm shift with our technology. Fusion procedures require cutting the bone to create room for the nerves to move their impingement, but you still have to leave enough bone to get through that bony bridge. If you do a wide decompression, it’s hard for the bridge to take hold.
We come in and tell the doctor look, you can perform as wide a decompression as you want. We don’t need a bony bridge. Make sure there’s no nerve root impingement — not today, not tomorrow. Put in our device and replicate the normal kinematics and physiology of the spine.
We knew going into the study that we would get good results. It came down to how well we executed. Jeff Withrow, our V.P. of Clinical and Reimbursement, ran a great study. He picked great sites and did great training. Surgeons chose the right patients. That reflects the potential of this technology when it’s used properly.
What does your rollout plan look like, once you receive approval?
Mr. Wehrly: It will be very focused. We have 37 sites plus another 10 physicians who went through cadaveric training. We’ll start with those institutions. Now that surgeons have seen the data, they’re calling patients. They have bought into the solution. In many of the institutions we’re working with, there are more doctors that we need to train and onboard. We’ll have three sales managers in the U.S. to find incremental distribution beyond those first 37.
I think the physicians in the know and coming out of residency or fellowship understand the benefits of motion and will be open to speaking with us. And at the end of the day, there’s no question that data is king.
The industry has been talking about motion preservation for decades. Several companies are seeking to differentiate in the space. What will the industry be saying about motion preservation in five years?
Mr. Sacher: There was a lot of excitement when motion preservation emerged in the early 2000s. My understanding has matured consistently with how the industry looks at motion preservation. Initially, I was naive and thought that anything that moves must be good. Then we started to see that’s not true. You can have products that move that are not designed to withstand the forces of the spine or sync with the spine. People went from initial excitement to realizing that it would not solve every problem, and it was overused or abused.
At some point, surgeons stepped back and said, we’re not sure if motion preservation makes sense, and it almost disappeared. It disappeared from the lumbar spine. But there was enough of an ember that when the flames were fanned, it managed to catch. The reason it caught was some really good companies had really good products. They did good research to show that in cervical disc replacement, you have a product that’s at least as good as fusion, if not better, when you look at two levels. Those companies learned from other companies’ mistakes and tried to maintain being on label and focusing on the right patient population. That evolution began what we’re seeing in the upswing of motion preservation.
I think we’re starting to see lumbar disc replacement also gather strength.
Five years from now, most surgeons will perform cervical disc replacements as their go-to procedure. Lumbar disc replacement will be a product of choice for the right patient. TOPS will be the gold standard for patients with stenosis and spondy.
What can we expect from Premia Spine in the next five years?
Mr. Sacher: The next big focus for us is the issue of reimbursement at the center level and the surgeon level.
Reimbursement will be great for the facilities because we’ll probably get the new technology add-on payment. Now we’re focused on surgeon payment. We want to make sure that we take care of the doctors, and they get paid what they deserve to get paid for this type of procedure.
We have more products in our pipeline that we’re developing, and we’ll get through clinicals to get those approved to expand indications for TOPS.
We’re going to stick with PMAs. We have another platform that we’re super excited about that we’ll start working on as well. It’s completely different from TOPS and has a huge market application.
What are some of the lessons that you’ve learned just from the PMA process?
Mr. Sacher: First, I highly recommend filing for a Breakthrough Device Designation if you can. Second, engage FDA in the interactive process. We did, and I think it was extremely helpful. Instead of just filing for a PMA, we broke our PMA into three modules. We worked with FDA through each of the three subsequent modules, starting with in vitro testing and biocompatibility, moving on to manufacturing and quality, and then finally moving into the clinical piece. That was a very good way of working with the agency. Engage the agency early and work with them closely. Those are the two big takeaways.
Also, make sure you raise enough money to finance the technology. PMAs are extremely expensive.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
CL
Carolyn LaWell is ORTHOWORLD's Chief Content Officer. She joined ORTHOWORLD in 2012 to oversee its editorial and industry education. She previously served in editor roles at B2B magazines and newspapers.







