
 Copy to clipboard
Copy to clipboard 
Premia Spine announced FDA approval of its Total Posterior Spine (TOPS) facet joint replacement system. TOPS previously earned the FDA’s Breakthrough Designation status and has now successfully completed its Premarket Approval PMA application.
Premia Spine undertook rigorous testing to receive FDA approval, including an Investigational Device Exemption (IDE) study to confirm the safety and efficacy of the system. The company’s pivotal FDA trial demonstrated that, at two years post-operation, the system outperformed fusion in treating grade 1 degenerative spondylolisthesis with stenosis. This accomplishment marks the TOPS System as the first lumbar spine implant to receive a superiority claim over lumbar fusion.
To address concerns relative to high reoperation rates, adjacent segment disease and additional adverse outcomes linked to spinal fusion, Premia’s prospective randomized controlled trial investigated the potential of TOPS facet arthroplasty as an alternative to TLIF fusion for treating this large patient population.
The study’s data revealed that the TOPS arthroplasty group significantly surpassed the TLIF fusion group in overall clinical success, with a 77% success rate compared to 24% respectively. While both arthroplasty and fusion methods stabilized the spine segment, the TOPS facet replacement preserved motion at the index level, leading to fewer complications and reoperations. Furthermore, patients who underwent TOPS arthroplasty reported improved outcomes in areas such as daily function and pain levels at all assessed time points. Notably, the gap in these improvements between the two groups expanded over time, becoming more pronounced from six months to 24 months postoperatively.
This landmark study aimed to demonstrate the clinical superiority of the TOPS System by randomizing patients at a 2:1 ratio to undergo either facet arthroplasty or TLIF fusion. The study was carried out across 37 centers, reinforcing its comprehensive and diverse surgeon and patient base. The primary outcome success was evaluated in a total of 168 subjects (115 TOPS arthroplasty and 53 TLIF fusion) at the two-year mark. The data revealed a significant advantage for the TOPS System over fusion in several areas: daily function improvement, with 95% success for TOPS arthroplasty versus 79% for TLIF fusion as per the Oswestry Disability Index; rates of new or worsening neural deficit, with 3% for TOPS arthroplasty versus 12% for TLIF fusion; rates of postoperative injections or surgical interventions, with 11% for TOPS arthroplasty versus 23% for TLIF fusion; and rates of motion or fusion failure, at a significantly lower 1% failure for TOPS arthroplasty versus 44% failure for TLIF fusion.
“The results of this study validate our hypothesis that TOPS lumbar facet arthroplasty yields a higher rate of clinical success and fewer revision procedures compared to fusion,” stated Ron Sacher, CEO of Premia Spine. “With FDA approval now secured, our priority shifts to ensuring access to the TOPS System for the countless individuals living with spinal stenosis and spondylolisthesis.”
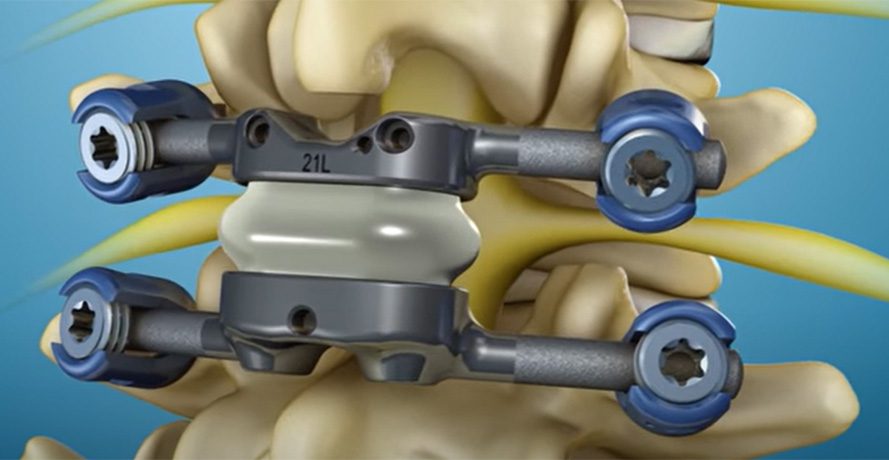
TOPS features two cross bars that affix to pedicle screws and a central motion mechanism, placed after bilateral facetectomies. The TOPS motion device is designed to allow widened decompression of the neural elements, stabilization of the spondylolisthesis, and preservation of motion in all ranges of motion.
TOPS stands as the first and only facet joint replacement system for the lumbar spine, designed to preserve mobility after decompression in patients suffering from lumbar spinal stenosis and degenerative spondylolisthesis.
Source: Premia Spine
Premia Spine announced FDA approval of its Total Posterior Spine (TOPS) facet joint replacement system. TOPS previously earned the FDA’s Breakthrough Designation status and has now successfully completed its Premarket Approval PMA application.
Premia Spine undertook rigorous testing to receive FDA approval, including an Investigational...
Premia Spine announced FDA approval of its Total Posterior Spine (TOPS) facet joint replacement system. TOPS previously earned the FDA’s Breakthrough Designation status and has now successfully completed its Premarket Approval PMA application.
Premia Spine undertook rigorous testing to receive FDA approval, including an Investigational Device Exemption (IDE) study to confirm the safety and efficacy of the system. The company’s pivotal FDA trial demonstrated that, at two years post-operation, the system outperformed fusion in treating grade 1 degenerative spondylolisthesis with stenosis. This accomplishment marks the TOPS System as the first lumbar spine implant to receive a superiority claim over lumbar fusion.
To address concerns relative to high reoperation rates, adjacent segment disease and additional adverse outcomes linked to spinal fusion, Premia’s prospective randomized controlled trial investigated the potential of TOPS facet arthroplasty as an alternative to TLIF fusion for treating this large patient population.
The study’s data revealed that the TOPS arthroplasty group significantly surpassed the TLIF fusion group in overall clinical success, with a 77% success rate compared to 24% respectively. While both arthroplasty and fusion methods stabilized the spine segment, the TOPS facet replacement preserved motion at the index level, leading to fewer complications and reoperations. Furthermore, patients who underwent TOPS arthroplasty reported improved outcomes in areas such as daily function and pain levels at all assessed time points. Notably, the gap in these improvements between the two groups expanded over time, becoming more pronounced from six months to 24 months postoperatively.
This landmark study aimed to demonstrate the clinical superiority of the TOPS System by randomizing patients at a 2:1 ratio to undergo either facet arthroplasty or TLIF fusion. The study was carried out across 37 centers, reinforcing its comprehensive and diverse surgeon and patient base. The primary outcome success was evaluated in a total of 168 subjects (115 TOPS arthroplasty and 53 TLIF fusion) at the two-year mark. The data revealed a significant advantage for the TOPS System over fusion in several areas: daily function improvement, with 95% success for TOPS arthroplasty versus 79% for TLIF fusion as per the Oswestry Disability Index; rates of new or worsening neural deficit, with 3% for TOPS arthroplasty versus 12% for TLIF fusion; rates of postoperative injections or surgical interventions, with 11% for TOPS arthroplasty versus 23% for TLIF fusion; and rates of motion or fusion failure, at a significantly lower 1% failure for TOPS arthroplasty versus 44% failure for TLIF fusion.
“The results of this study validate our hypothesis that TOPS lumbar facet arthroplasty yields a higher rate of clinical success and fewer revision procedures compared to fusion,” stated Ron Sacher, CEO of Premia Spine. “With FDA approval now secured, our priority shifts to ensuring access to the TOPS System for the countless individuals living with spinal stenosis and spondylolisthesis.”
TOPS features two cross bars that affix to pedicle screws and a central motion mechanism, placed after bilateral facetectomies. The TOPS motion device is designed to allow widened decompression of the neural elements, stabilization of the spondylolisthesis, and preservation of motion in all ranges of motion.
TOPS stands as the first and only facet joint replacement system for the lumbar spine, designed to preserve mobility after decompression in patients suffering from lumbar spinal stenosis and degenerative spondylolisthesis.
Source: Premia Spine

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







