
 Copy to clipboard
Copy to clipboard 
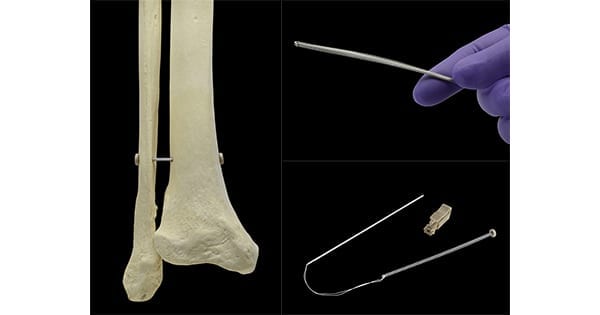
Panther Orthopedics announced exceptional mid-term clinical outcomes for PUMA System™, the first and only FDA-cleared superelastic syndesmosis device featuring a spring-like design for initial and continuous compression without the need to over-tension.
PUMA received FDA 510(k) clearance in 2018, and the company has since closed financing to support the system’s clinical use.
The device offers an alternative to existing screw or endobutton type fixation devices. Over-compression of the distal tibiofibular syndesmosis may lead to crushing of the microvasculature, malreduction and a stiff joint. In PUMA’s initial commercialization, over 50 patients were followed for procedural safety and healing response via postoperative radiographic and clinical evaluations for up to 22 months.
“We are excited about our clinical results demonstrating excellent joint healing and early return to mobilization and physical activity. We confirmed satisfactory radiographic syndesmosis healing with reduction in the ankle mortise in 100% of these patients and there was no evidence of lysis, device migration or syndesmotic widening. Additionally, there were no infections, soft tissue complications or devices requiring removal or revision surgeries,” said Kathy Stecco, M.D., CEO and Co-founder of Panther Orthopedics.
Panther Orthopedics announced exceptional mid-term clinical outcomes for PUMA System™, the first and only FDA-cleared superelastic syndesmosis device featuring a spring-like design for initial and continuous compression without the need to over-tension.
PUMA received FDA 510(k) clearance in 2018, and the company has since closed...
Panther Orthopedics announced exceptional mid-term clinical outcomes for PUMA System™, the first and only FDA-cleared superelastic syndesmosis device featuring a spring-like design for initial and continuous compression without the need to over-tension.
PUMA received FDA 510(k) clearance in 2018, and the company has since closed financing to support the system’s clinical use.
The device offers an alternative to existing screw or endobutton type fixation devices. Over-compression of the distal tibiofibular syndesmosis may lead to crushing of the microvasculature, malreduction and a stiff joint. In PUMA’s initial commercialization, over 50 patients were followed for procedural safety and healing response via postoperative radiographic and clinical evaluations for up to 22 months.
“We are excited about our clinical results demonstrating excellent joint healing and early return to mobilization and physical activity. We confirmed satisfactory radiographic syndesmosis healing with reduction in the ankle mortise in 100% of these patients and there was no evidence of lysis, device migration or syndesmotic widening. Additionally, there were no infections, soft tissue complications or devices requiring removal or revision surgeries,” said Kathy Stecco, M.D., CEO and Co-founder of Panther Orthopedics.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







