
 Copy to clipboard
Copy to clipboard 
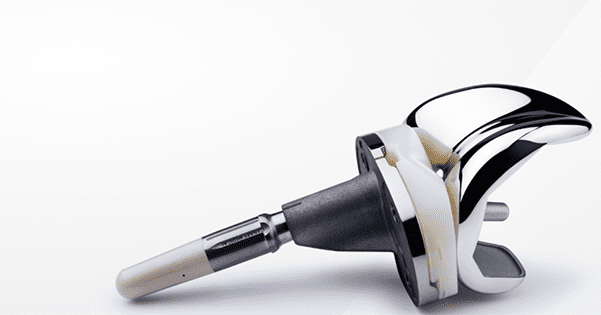
Zimmer Biomet received FDA De Novo classification for its Persona IQ tibial extension, a smart implant for total knee replacement. The Persona IQ not only enhances Zimmer Biomet’s knee portfolio, but it amplifies the competition amongst joint replacement companies seeking to track patient data throughout the episode of care.
Persona IQ at a Glance
The updated implant pairs Zimmer Biomet’s Persona Personalized Knee with Canary Medical’s proprietary CANARY canturio te tibial extension. CANARY canturio te uses the same material and technology found in pacemakers and other implantable cardiac devices and includes a sensor that is powered by a battery with a lifespan of up to 10 years.
The technology records and wirelessly transmits information to a patient’s base station and then is shared with surgeons through a secure cloud-based platform. Multiple recovery indicators are captured, including the patient’s range of motion, step count, walking speed and other gait measures to track passive data instead of relying solely on patient-reported information.
Sensors have been studied, discussed and used in orthopedics for decades. While they’re gaining adoption for use in surgical procedures and to track patient recovery through wearables, implantable sensors are a significant next step for orthopedics.
Canary Medical’s technology underwent almost a decade of development before receiving FDA Breakthrough Device Designation in 2019.
“Persona IQ reflects our shared belief that automatic, reliable and accurate data collection and analysis represents the future of orthopedic care,” said William L. Hunter, M.D., CEO of Canary Medical.
Enhanced Ecosystem of Products
Persona IQ is the newest component to Zimmer Biomet’s ZBEdge, the company’s suite of connected digital and robotic technologies that include ROSA robotics and mymobility. Data from the technologies are incorporated into Zimmer Biomet’s intelligence platform, OrthoIntel, and then provided to surgeons to inform patient care decisions.
Of course, Zimmer Biomet is not alone in this data pursuit.
Large- and medium-sized joint replacement companies see collecting data and disseminating that analyzed data to personalize and better inform procedures as a crucial part of their solutions portfolios moving forward. These technologies range from surgical risk assessment tools, management tools to connect the patient and surgical team pre- and post-surgery, wearable devices and, of course, surgical planning, navigation and robotic systems.
Part of the attraction is that enabling and digital technology often provides companies a recurring revenue stream, as physicians are incentivized to use a company’s technology alongside their implants. This became even clearer in the last year and a half as companies noted that enabling technology has mainly been “pandemic-proof” in terms of sales, even though knee and hip implant sales were well below historical performance.
Since he was appointed President and CEO of Zimmer Biomet, Bryan Hanson has touted enabling technology and this digital connectedness. He once said that these technologies would “bend the growth curve” of orthopedics.
Zimmer Biomet has launched the most comprehensive portfolio to date. But its main competitors – Stryker, DePuy Synthes and Smith+Nephew – are each building out connected platforms. Joint replacement companies like Medacta, DJO, Exactech, Corin and MicroPort Orthopedics have also internally developed or collaborated with other companies to incorporate technology that informs surgical procedures or tracks patient recovery.
The $9 billion knee replacement market has been a primary focus for connected digital portfolios. It’s expected that hip and extremities joint replacement will be next once the foundation for these tools has been established.
“As the newest component of ZBEdge, Persona IQ advances our vision of creating a seamlessly connected suite of digital health and robotic technologies to deliver objective data to clinicians throughout the surgical journey,” Mr. Hanson said. “Following a recent expansion of our partnership with Canary Medical, we now expect that Persona IQ will be the first in a broader portfolio of smart implant technologies in various orthopedic surgery applications.”
Future of Smart Implants
In announcing Persona IQ’s De Novo clearance, executives from Zimmer Biomet and Canary Medical acknowledged an extension of their partnership to look at additional orthopedic applications. Zimmer Biomet previously provided Canary Medical a $20 Million venture loan facility to assist with the final FDA steps and development of new applications for its sensors.
We interviewed Dr. Hunter earlier this year, and he made several notable comments about the future of smart implants. First, Canary Medical’s technology is designed to allow it to be developed for a host of orthopedic implants, including hips, shoulders and fracture fixation devices. Second, FDA expected self-reporting implants to be the next evolution for joint replacement and was engaged and interactive throughout the process. Like any novel device, FDA now has a sense of a framework for clearing similar devices in orthopedics. Third, market clearance and access are just the beginning. To indeed provide predictive value for physicians, it will take data from thousands of patients and time and resources to deliver on its value.
Still, a smart knee is pretty remarkable.
Carolyn LaWell is ORTHOWORLD’s Chief Content Officer.
Zimmer Biomet received FDA De Novo classification for its Persona IQ tibial extension, a smart implant for total knee replacement. The Persona IQ not only enhances Zimmer Biomet’s knee portfolio, but it amplifies the competition amongst joint replacement companies seeking to track patient data throughout the episode of care.
Persona IQ at a...
Zimmer Biomet received FDA De Novo classification for its Persona IQ tibial extension, a smart implant for total knee replacement. The Persona IQ not only enhances Zimmer Biomet’s knee portfolio, but it amplifies the competition amongst joint replacement companies seeking to track patient data throughout the episode of care.
Persona IQ at a Glance
The updated implant pairs Zimmer Biomet’s Persona Personalized Knee with Canary Medical’s proprietary CANARY canturio te tibial extension. CANARY canturio te uses the same material and technology found in pacemakers and other implantable cardiac devices and includes a sensor that is powered by a battery with a lifespan of up to 10 years.
The technology records and wirelessly transmits information to a patient’s base station and then is shared with surgeons through a secure cloud-based platform. Multiple recovery indicators are captured, including the patient’s range of motion, step count, walking speed and other gait measures to track passive data instead of relying solely on patient-reported information.
Sensors have been studied, discussed and used in orthopedics for decades. While they’re gaining adoption for use in surgical procedures and to track patient recovery through wearables, implantable sensors are a significant next step for orthopedics.
Canary Medical’s technology underwent almost a decade of development before receiving FDA Breakthrough Device Designation in 2019.
“Persona IQ reflects our shared belief that automatic, reliable and accurate data collection and analysis represents the future of orthopedic care,” said William L. Hunter, M.D., CEO of Canary Medical.
Enhanced Ecosystem of Products
Persona IQ is the newest component to Zimmer Biomet’s ZBEdge, the company’s suite of connected digital and robotic technologies that include ROSA robotics and mymobility. Data from the technologies are incorporated into Zimmer Biomet’s intelligence platform, OrthoIntel, and then provided to surgeons to inform patient care decisions.
Of course, Zimmer Biomet is not alone in this data pursuit.
Large- and medium-sized joint replacement companies see collecting data and disseminating that analyzed data to personalize and better inform procedures as a crucial part of their solutions portfolios moving forward. These technologies range from surgical risk assessment tools, management tools to connect the patient and surgical team pre- and post-surgery, wearable devices and, of course, surgical planning, navigation and robotic systems.
Part of the attraction is that enabling and digital technology often provides companies a recurring revenue stream, as physicians are incentivized to use a company’s technology alongside their implants. This became even clearer in the last year and a half as companies noted that enabling technology has mainly been “pandemic-proof” in terms of sales, even though knee and hip implant sales were well below historical performance.
Since he was appointed President and CEO of Zimmer Biomet, Bryan Hanson has touted enabling technology and this digital connectedness. He once said that these technologies would “bend the growth curve” of orthopedics.
Zimmer Biomet has launched the most comprehensive portfolio to date. But its main competitors – Stryker, DePuy Synthes and Smith+Nephew – are each building out connected platforms. Joint replacement companies like Medacta, DJO, Exactech, Corin and MicroPort Orthopedics have also internally developed or collaborated with other companies to incorporate technology that informs surgical procedures or tracks patient recovery.
The $9 billion knee replacement market has been a primary focus for connected digital portfolios. It’s expected that hip and extremities joint replacement will be next once the foundation for these tools has been established.
“As the newest component of ZBEdge, Persona IQ advances our vision of creating a seamlessly connected suite of digital health and robotic technologies to deliver objective data to clinicians throughout the surgical journey,” Mr. Hanson said. “Following a recent expansion of our partnership with Canary Medical, we now expect that Persona IQ will be the first in a broader portfolio of smart implant technologies in various orthopedic surgery applications.”
Future of Smart Implants
In announcing Persona IQ’s De Novo clearance, executives from Zimmer Biomet and Canary Medical acknowledged an extension of their partnership to look at additional orthopedic applications. Zimmer Biomet previously provided Canary Medical a $20 Million venture loan facility to assist with the final FDA steps and development of new applications for its sensors.
We interviewed Dr. Hunter earlier this year, and he made several notable comments about the future of smart implants. First, Canary Medical’s technology is designed to allow it to be developed for a host of orthopedic implants, including hips, shoulders and fracture fixation devices. Second, FDA expected self-reporting implants to be the next evolution for joint replacement and was engaged and interactive throughout the process. Like any novel device, FDA now has a sense of a framework for clearing similar devices in orthopedics. Third, market clearance and access are just the beginning. To indeed provide predictive value for physicians, it will take data from thousands of patients and time and resources to deliver on its value.
Still, a smart knee is pretty remarkable.
Carolyn LaWell is ORTHOWORLD’s Chief Content Officer.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
CL
Carolyn LaWell is ORTHOWORLD's Chief Content Officer. She joined ORTHOWORLD in 2012 to oversee its editorial and industry education. She previously served in editor roles at B2B magazines and newspapers.







