
 Copy to clipboard
Copy to clipboard 
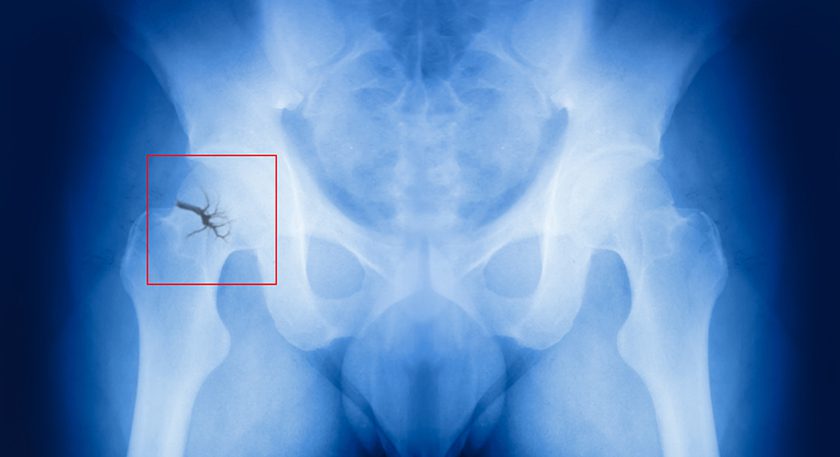
X-Bolt Orthopedics seeks to enter the trauma market as disruptors when surgeons perform the first surgeries with the company’s products later this month. The Dublin-headquartered entity has spent more than a decade collecting data and perfecting its first product, the PRO-X1 Trochanetic Nail, which provides a novel solution for patients with osteoporotic hip fractures.
While in practice, Brian Thornes, M.D., an orthopedic surgeon, X-Bolt founder and Chief Executive Officer, saw that when hip fractures with poor bone quality were treated with screws, the patient would experience cut-out, where the screw loses fixation and enters the hip joint. The solution, Dr. Thornes thought, was an expandable bolt that could provide greater rotational stability and anchorage into the bone. The differentiator from other expandable bolts on the market is that X-Bolt’s device is mechanically reversible.
In 2023, X-Bolt received FDA 510(k) clearance for PRO-X1 and raised $4 million in a Series A that was allocated to build the team and inventory for their first cases. The company plans to do a limited launch in 20 hospitals across Florida and the East Coast starting at the end of March. Dr. Thornes said they have demand in over 50 hospitals and will seek to raise additional capital in the next year to build commercialization efforts.
Dr. Thornes also developed and licensed the TightRope Ankle to Arthrex in 2003. We spoke with him about his latest development and commercialization journey.
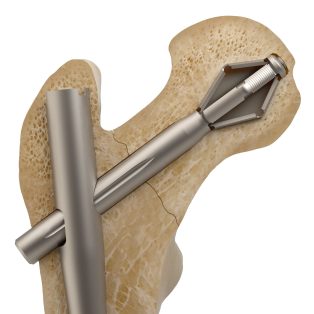
The PRO-X1 Trochanetic Nail provides a novel solution for patients with osteoporotic hip fractures. The expandable bolt could provide greater rotational stability and anchorage into the bone.
You founded the company in 2007. How has X-Bolt’s technology evolved since your first iterations?
Dr. Thornes: The base concept is still the same, but we changed our material from stainless steel to titanium.
We’ve also made little innovation wins with our instrumentation. We have a Metro-Jig, which is a long and flexible curved jig. The surgeon can put the bolt through the jig and operate all the instruments from outside the body, which takes five to 10 minutes off the operating time. The Metro-Jig is particularly good for obese patients because it doesn’t require you to poke around in soft tissues.
We also have an instrument called the Bone Crusher. Its primary function is to create a little star cavity for the device’s wings, but the real win is that once you punch it and it opens up, you then have full control of the femoral head and can dial in the reduction. The surgeon can get a great feel for whether the bone is hard or soft.
A lot of thought has gone into nearly every aspect of surgery. It has been rewarding for people to realize that deep thought has gone into solving problems, big and small.
You prioritized clinical trials and collected a significant amount of data prior to the FDA 510(k) submission for the PRO-X1. Why was that important to you?
Dr. Thornes: My experiences as a surgeon and with the TightRope guided our path. TightRope was slow to take off in its first decade on the market because there wasn’t good clinical data to back the product. I wanted clinical data for this product.
We are lucky to be next door to the UK, where their socialized medicine lends itself to running large trials. The WHITE1 study had 100 patients and WHITE4 had 1,140 patients. We drove those studies to collect a lot of clinical data. If you told me at the beginning of the trial that we were going to have a cut-out rate of 0.8%, I wouldn’t have believed you. The rate is less than half of the 2.5% that is typically reported in the literature.
We also knew that the market was moving toward troch nails. After our trials, we regrouped and developed a titanium troch nail. Those efforts culminated in our FDA clearance.
What sets us apart is that we have a bank of clinical data. We’re able to go to hospital VACs and show through data that the product’s concept is safe and reliable, and surgeons love it.
Who will be your main competitors in the trauma market?
Dr. Thornes: We’re setting ourselves up as disruptors. I don’t know what the big companies will think. We’re a novelty at this stage; we’re not going to take a considerable amount of revenue from them. We’re predicting $2 million in revenue for 2024 and $13 million in 2025. We’re focused on getting a full house of products and becoming profitable quickly.
What other products do you have in the pipeline?
Dr. Thornes: We know that having a single solution isn’t enough, so we’re developing additional hip fracture products. We have a Petite Pro-X1 nail to cover the small-stature population, particularly Asians and Hispanics. Our technology will be the better option for the petite population. The big win with our device is that the bolt expands on the far side of the nail, and you don’t lose as much anchorage as you would with screws. When you downsize a nail that has a lag screw in it, you lose a lot of the thread diameter. We don’t have that issue with an expanding bolt.
We also have a dynamic plating product and a retrograde nail with a double ended X-Bolt in the pipeline. We’re setting ourselves up as a trauma company and will have products for the femur, humerus and tibia.
We also know that screw loosening is a problem in the osteoporotic spine, so we produced a DragonBolt Pedicle Screw Fixation System. Our cyclic load testing shows that it performs 400% better than the standard pedicle screw. We will look for a licensing partner to commercialize it.
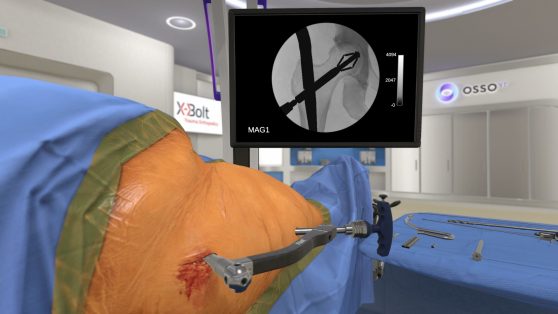
X-Bolt Orthopedics worked with Osso VR to create surgical training for the use of PRO-X1.
What are some of the broader trends you’re hearing from surgeons?
Dr. Thornes: A lot of the chats are about robotics, AR and enabling technologies. We’ve partnered with Osso VR on a virtual reality application, which is fantastic and so realistic. I’m looking forward to having it as a learning tool for surgeons. Sometimes, new technology needs to be demystified. I think VR will make the adoption of our technology quicker because surgeons will put on the headset, go through the surgery in 10 minutes and realize it is easy.
VR is in its infancy now. In the years to come, it will be commonplace for medical students and residents to use it to learn different techniques. We wanted to be at the forefront of that education.
What lessons have you learned from bringing multiple technologies to market?
Dr. Thornes: It has to be fun, and I’ve enjoyed this. As a surgeon, I know what it’s like to be in a tough place in the O.R. A high volume of hip fractures can be daily drudgery. We aim to bring fun back into orthopedics. I like working with gadgets, as do a lot of other orthopedic surgeons. I’ve invented cool gadgets like the TightRope and X-Bolt, and I hope surgeons enjoy using them. The startup life has its highs and lows, but we do what we do because it’s enjoyable.
X-Bolt Orthopedics seeks to enter the trauma market as disruptors when surgeons perform the first surgeries with the company’s products later this month. The Dublin-headquartered entity has spent more than a decade collecting data and perfecting its first product, the PRO-X1 Trochanetic Nail, which provides a novel solution for patients with...
X-Bolt Orthopedics seeks to enter the trauma market as disruptors when surgeons perform the first surgeries with the company’s products later this month. The Dublin-headquartered entity has spent more than a decade collecting data and perfecting its first product, the PRO-X1 Trochanetic Nail, which provides a novel solution for patients with osteoporotic hip fractures.
While in practice, Brian Thornes, M.D., an orthopedic surgeon, X-Bolt founder and Chief Executive Officer, saw that when hip fractures with poor bone quality were treated with screws, the patient would experience cut-out, where the screw loses fixation and enters the hip joint. The solution, Dr. Thornes thought, was an expandable bolt that could provide greater rotational stability and anchorage into the bone. The differentiator from other expandable bolts on the market is that X-Bolt’s device is mechanically reversible.
In 2023, X-Bolt received FDA 510(k) clearance for PRO-X1 and raised $4 million in a Series A that was allocated to build the team and inventory for their first cases. The company plans to do a limited launch in 20 hospitals across Florida and the East Coast starting at the end of March. Dr. Thornes said they have demand in over 50 hospitals and will seek to raise additional capital in the next year to build commercialization efforts.
Dr. Thornes also developed and licensed the TightRope Ankle to Arthrex in 2003. We spoke with him about his latest development and commercialization journey.

The PRO-X1 Trochanetic Nail provides a novel solution for patients with osteoporotic hip fractures. The expandable bolt could provide greater rotational stability and anchorage into the bone.
You founded the company in 2007. How has X-Bolt’s technology evolved since your first iterations?
Dr. Thornes: The base concept is still the same, but we changed our material from stainless steel to titanium.
We’ve also made little innovation wins with our instrumentation. We have a Metro-Jig, which is a long and flexible curved jig. The surgeon can put the bolt through the jig and operate all the instruments from outside the body, which takes five to 10 minutes off the operating time. The Metro-Jig is particularly good for obese patients because it doesn’t require you to poke around in soft tissues.
We also have an instrument called the Bone Crusher. Its primary function is to create a little star cavity for the device’s wings, but the real win is that once you punch it and it opens up, you then have full control of the femoral head and can dial in the reduction. The surgeon can get a great feel for whether the bone is hard or soft.
A lot of thought has gone into nearly every aspect of surgery. It has been rewarding for people to realize that deep thought has gone into solving problems, big and small.
You prioritized clinical trials and collected a significant amount of data prior to the FDA 510(k) submission for the PRO-X1. Why was that important to you?
Dr. Thornes: My experiences as a surgeon and with the TightRope guided our path. TightRope was slow to take off in its first decade on the market because there wasn’t good clinical data to back the product. I wanted clinical data for this product.
We are lucky to be next door to the UK, where their socialized medicine lends itself to running large trials. The WHITE1 study had 100 patients and WHITE4 had 1,140 patients. We drove those studies to collect a lot of clinical data. If you told me at the beginning of the trial that we were going to have a cut-out rate of 0.8%, I wouldn’t have believed you. The rate is less than half of the 2.5% that is typically reported in the literature.
We also knew that the market was moving toward troch nails. After our trials, we regrouped and developed a titanium troch nail. Those efforts culminated in our FDA clearance.
What sets us apart is that we have a bank of clinical data. We’re able to go to hospital VACs and show through data that the product’s concept is safe and reliable, and surgeons love it.
Who will be your main competitors in the trauma market?
Dr. Thornes: We’re setting ourselves up as disruptors. I don’t know what the big companies will think. We’re a novelty at this stage; we’re not going to take a considerable amount of revenue from them. We’re predicting $2 million in revenue for 2024 and $13 million in 2025. We’re focused on getting a full house of products and becoming profitable quickly.
What other products do you have in the pipeline?
Dr. Thornes: We know that having a single solution isn’t enough, so we’re developing additional hip fracture products. We have a Petite Pro-X1 nail to cover the small-stature population, particularly Asians and Hispanics. Our technology will be the better option for the petite population. The big win with our device is that the bolt expands on the far side of the nail, and you don’t lose as much anchorage as you would with screws. When you downsize a nail that has a lag screw in it, you lose a lot of the thread diameter. We don’t have that issue with an expanding bolt.
We also have a dynamic plating product and a retrograde nail with a double ended X-Bolt in the pipeline. We’re setting ourselves up as a trauma company and will have products for the femur, humerus and tibia.
We also know that screw loosening is a problem in the osteoporotic spine, so we produced a DragonBolt Pedicle Screw Fixation System. Our cyclic load testing shows that it performs 400% better than the standard pedicle screw. We will look for a licensing partner to commercialize it.

X-Bolt Orthopedics worked with Osso VR to create surgical training for the use of PRO-X1.
What are some of the broader trends you’re hearing from surgeons?
Dr. Thornes: A lot of the chats are about robotics, AR and enabling technologies. We’ve partnered with Osso VR on a virtual reality application, which is fantastic and so realistic. I’m looking forward to having it as a learning tool for surgeons. Sometimes, new technology needs to be demystified. I think VR will make the adoption of our technology quicker because surgeons will put on the headset, go through the surgery in 10 minutes and realize it is easy.
VR is in its infancy now. In the years to come, it will be commonplace for medical students and residents to use it to learn different techniques. We wanted to be at the forefront of that education.
What lessons have you learned from bringing multiple technologies to market?
Dr. Thornes: It has to be fun, and I’ve enjoyed this. As a surgeon, I know what it’s like to be in a tough place in the O.R. A high volume of hip fractures can be daily drudgery. We aim to bring fun back into orthopedics. I like working with gadgets, as do a lot of other orthopedic surgeons. I’ve invented cool gadgets like the TightRope and X-Bolt, and I hope surgeons enjoy using them. The startup life has its highs and lows, but we do what we do because it’s enjoyable.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
CL
Carolyn LaWell is ORTHOWORLD's Chief Content Officer. She joined ORTHOWORLD in 2012 to oversee its editorial and industry education. She previously served in editor roles at B2B magazines and newspapers.