
 Copy to clipboard
Copy to clipboard 
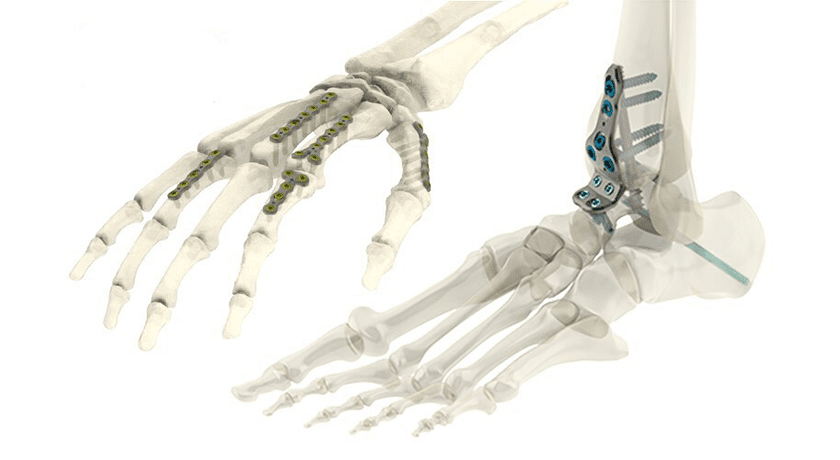
Tyber Medical was granted FDA 510(k) clearance that expands its anatomical plate portfolio. The company’s latest FDA clearance extends the breadth of its Anatomical Plating System with 391 additional plate configurations, including 100 new plates targeting foot, ankle and wrist indications.
Tyber Medical’s Anatomical Plating System was also deemed safe to carry MR Conditional labeling by FDA.
Tyber Medical’s 510(k)-cleared plating system is indicated to treat a comprehensive range of deformity, trauma and degenerative conditions of the wrist, foot, ankle and long bones. The MR Conditional term allows Tyber Medical’s Anatomical Plating System to be safely used in the MRI environment under certain tested conditions.
According to Tyber Medical’s Director of Regulatory Affairs Lisa Boyle, the company has vigorously worked to meet the requirements necessary to gain this important designation from the FDA. “Over the past two years, the FDA has raised the bar for the industry to meet the MR compatibility requirements,” said Boyle. “Our vertically integrated team has gone above and beyond to meet these requirements, and is very excited that our Anatomical Plating System has demonstrated safety in the MR environment, permitting us to label our system as MR Conditional.”
Tyber Medical officially launched its Anatomical Plating System in 2020, when the product line received its initial 510(k) clearance from FDA.
Source: Tyber Medical LLC
Tyber Medical was granted FDA 510(k) clearance that expands its anatomical plate portfolio. The company's latest FDA clearance extends the breadth of its Anatomical Plating System with 391 additional plate configurations, including 100 new plates targeting foot, ankle and wrist indications.
Tyber Medical's Anatomical Plating System was also...
Tyber Medical was granted FDA 510(k) clearance that expands its anatomical plate portfolio. The company’s latest FDA clearance extends the breadth of its Anatomical Plating System with 391 additional plate configurations, including 100 new plates targeting foot, ankle and wrist indications.
Tyber Medical’s Anatomical Plating System was also deemed safe to carry MR Conditional labeling by FDA.
Tyber Medical’s 510(k)-cleared plating system is indicated to treat a comprehensive range of deformity, trauma and degenerative conditions of the wrist, foot, ankle and long bones. The MR Conditional term allows Tyber Medical’s Anatomical Plating System to be safely used in the MRI environment under certain tested conditions.
According to Tyber Medical’s Director of Regulatory Affairs Lisa Boyle, the company has vigorously worked to meet the requirements necessary to gain this important designation from the FDA. “Over the past two years, the FDA has raised the bar for the industry to meet the MR compatibility requirements,” said Boyle. “Our vertically integrated team has gone above and beyond to meet these requirements, and is very excited that our Anatomical Plating System has demonstrated safety in the MR environment, permitting us to label our system as MR Conditional.”
Tyber Medical officially launched its Anatomical Plating System in 2020, when the product line received its initial 510(k) clearance from FDA.
Source: Tyber Medical LLC

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







