
 Copy to clipboard
Copy to clipboard 
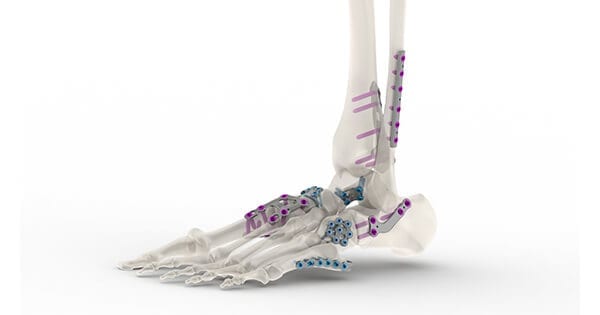
Tyber Medical was granted FDA 510(k) clearance to market its new line of foot and ankle plating systems, including over 42 different indication-specific anatomical plating families.
The company will launch the first phase of plates in 1H21, and is pursuing CE Mark Approval, which is expected in 2H21.
The systems feature a 30-degree, multi-angle, locking mechanism for stability between the plate and screws. A modular tray delivery system is customizable with a color-coded layout that is consistent with the surgical flow. The sterile and non-sterile plates include a combination of material and surface modification to significantly increase fatigue resistance.
“Our system is designed to minimize soft tissue trauma during the procedure,” said Melissa Coale, Tyber Medical senior engineer. “Our plates feature geometry to reduce overall prominence, especially in areas with minimal soft tissue coverage.”
Tyber Medical CEO and President, Jeff Tyber, said, “Our team has developed a robust method linking intrinsic design and ease of use for a variety of surgical indications. We expect to expand this methodology beyond the lower extremities and into all areas of the skeletal anatomy.”
Tyber Medical was granted FDA 510(k) clearance to market its new line of foot and ankle plating systems, including over 42 different indication-specific anatomical plating families.
The company will launch the first phase of plates in 1H21, and is pursuing CE Mark Approval, which is expected in 2H21.
The systems feature a 30-degree,...
Tyber Medical was granted FDA 510(k) clearance to market its new line of foot and ankle plating systems, including over 42 different indication-specific anatomical plating families.
The company will launch the first phase of plates in 1H21, and is pursuing CE Mark Approval, which is expected in 2H21.
The systems feature a 30-degree, multi-angle, locking mechanism for stability between the plate and screws. A modular tray delivery system is customizable with a color-coded layout that is consistent with the surgical flow. The sterile and non-sterile plates include a combination of material and surface modification to significantly increase fatigue resistance.
“Our system is designed to minimize soft tissue trauma during the procedure,” said Melissa Coale, Tyber Medical senior engineer. “Our plates feature geometry to reduce overall prominence, especially in areas with minimal soft tissue coverage.”
Tyber Medical CEO and President, Jeff Tyber, said, “Our team has developed a robust method linking intrinsic design and ease of use for a variety of surgical indications. We expect to expand this methodology beyond the lower extremities and into all areas of the skeletal anatomy.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







