

 Copy to clipboard
Copy to clipboard 
Trauma remains one of the more attractive markets for orthopaedic device companies due to demographics, ripeness for innovation and an increased focus on lower extremities care, to name just three factors. These strengths have led companies of all sizes—established orthopaedic players and startups alike—to enter the trauma market. We think this dynamic will make trauma one of the more exciting markets to watch in coming years.
Nearly 200 companies worldwide sell implants and instruments for internal and external fracture repair. In 2017 the market reached $6.9 billion, +4.6% vs. 2016, according to our estimates published in THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT®.
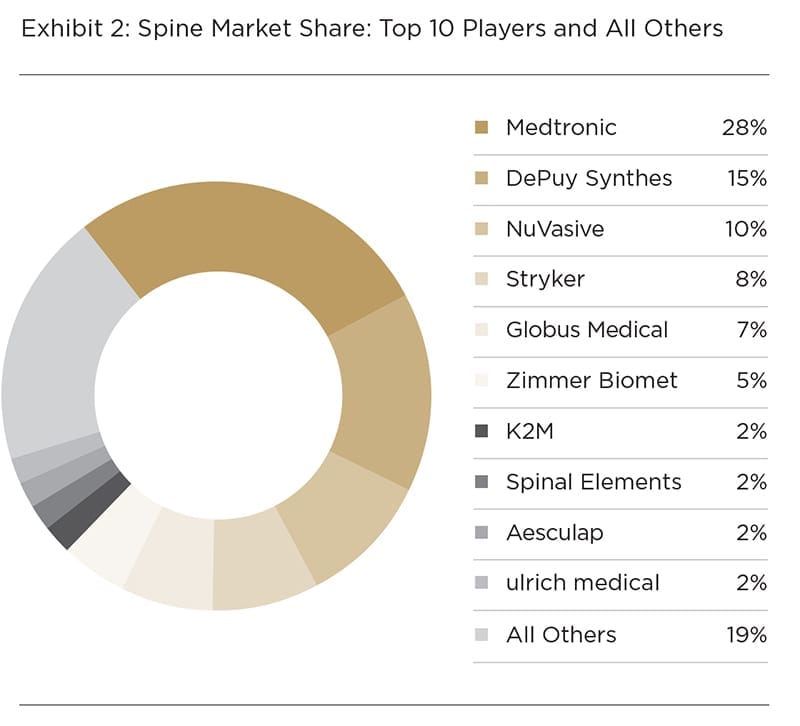
As can be seen in Exhibits 1 and 2, DePuy Synthes is the dominant company in the segment, posting ~$2.5 billion in revenue in 2017, which accounted for 36% market share—a 16% lead over second place Stryker. DePuy Synthes and Stryker are well-positioned to maintain their hold on the market. Of interest is the amount of activity taking place among mid-tier and smaller trauma players vying for market share.
Exhibit 1: Trauma Sales Performance: Top 10 Players and All Others ($Millions)
| 2017 | 2016 | $ Change | % Change | |
| DePuy Synthes | $2,490.9 | $2,439.1 | $51.8 | 2.1% |
| Stryker | $1,378.1 | $1,265.8 | $112.3 | 8.9% |
| Zimmer Biomet | $560.2 | $536.3 | $23.9 | 4.5% |
| Smith & Nephew | $441.0 | $422.8 | $18.3 | 4.3% |
| Top-Tier Players Total | $4,870.2 | $4,663.5 | $206.7 | 4.4% |
| Wright Medical | $282.9 | $267.1 | $15.8 | 5.9% |
| Acumed | $200.0 | $189.4 | $10.6 | 5.6% |
| Aesculap | $123.2 | $117.9 | $5.3 | 4.5% |
| Osteomed | $109.7 | $104.5 | $5.2 | 5.0% |
| Orthofix | $103.2 | $102.7 | $0.5 | 0.5% |
| Medartis | $94.3 | $83.7 | $10.6 | 12.7% |
| Next-Tier Players Total | $913.3 | $865.3 | $48.0 | 5.6% |
| ~180 companies with revenue below $99MM | $1,136.5 | $1,086.0 | $50.5 | 4.7% |
| Total | $6,920.0 | $6,615.2 | $304.8 | 4.6% |
Exhibit 2: Trauma Market Share: Top 10 Players and All Others
In 2017, we noted 11 companies that received their first FDA 510(k) in trauma, and we identified an additional five companies in 1Q18. (See Exhibit 3 at the close of this article.) Most notably, spine company Globus Medical, which received clearance for products to treat shoulder, wrist and ankle fractures, and Germany-headquartered Wittenstein Intens, a multinational, multi-industry company that entered the U.S. market with its limb lengthening technology.
Additionally, in 2017, three notable acquisitions were made in the space. Arthrex purchased Sonoma Orthopedic Products, developer of wrist and clavicle implants. Medartis, prior to going public in 2018, acquired Mimedis, a Swiss startup that developed preoperative planning solutions. Osteomed bought OT Medical’s InstaFix shape memory staple systems.
We project the trauma market to grow in the high 4% range for each of the next five years, surpassing $8.7 billion by 2022.
The activity propelling this growth includes:
- A focus on lower extremity products. Many companies have made investments in this space, and we expect that the lifecycle of these products will play out over coming years, making this portion of the market worth watching.
- Prioritization of large companies. With patient demographics and expanding indications in mind, larger players will continue to grow their trauma portfolios to take advantage of cross-selling opportunities with other segments in which they play. Think DePuy Synthes and hip, and Wright Medical and extremities.
- Players of all sizes and from other segments will continue to make a concerted push in trauma. NuVasive and Globus Medical, traditionally spine companies, have entered the field. Arthrex, a traditionally arthroscopy/soft tissue repair company, has made acquisitions and received a handful of clearances in the last two years.
Exhibit 3: Companies Gaining First FDA 510(k) Clearances for Trauma Devices in 2017 and 1Q18
AKROS MEDICAL – Fibulink Syndesmosis Repair Kit
- Anchor to provide fixation between tibia and fibula after ankle syndesmosis
- Raised $610K in 4Q15 from private investors
ANJON HOLDINGS – Anjon Bermer Halo System
- Halo system intended to provide cervical spine immobilization for treatment of cervical trauma
- Bone anchorage elements include titanium threaded skull pins, aluminum crown and rod superstructure and polymer lined vest
EXOTOE – ExoToe Staple
- Intended for fracture, osteotomy fixation and joint arthrodesis of the foot in conjunction with k-wire fixation
- Provided in a sterile pack
EXSOMED – ArcPhix and ArrowPhix Small Bone Screw Systems
- Stainless steel compression screws for fixation of small bones, bone fragments, osteotomies (not soft tissue)
- Provided in a sterile, single-use kit
GLOBUS MEDICAL – Anthem Fracture System
- Made from titanium alloy, cobalt chromium, molybdenum alloy or stainless steel
- Comprises a Proximal Humerus Plating System for shoulder fractures, Clavicle Plating System, Comprehensive Distal Radius Fracture System, Proximal Tibia Plating, Comprehensive Ankle Fracture System and a Small Fragment Plating System
INTRAFUSE – Flex-Thread Clavicle Pin
- Stainless steel and PEEK, comprises a flexible threaded distal tip to engage the curved medial portion of the clavicle and a proximal cross screw to support stability and fixation to the lateral portion of the bone
- Portfolio company of incubator Surgical Frontiers that will focus on less invasive intramedullary fracture fixation of clavicle, radius, ulna and fibula
ORTHOVESTMENTS – Orbitum Bone Staple Implant X and VI
- Titanium-based implants ORBITUM X and VI Bone Staple indicated for hand and foot fractures
- ORTBITUM X and VI Implants intended for foot fractures, hallux valgus and primus varus treatment
ORTHOXEL – Apex Tibial Nail
- Reportedly offers the largest range of locking options of any intramedullary nail, and features a micromotion locking mode to support controlled axial movement with torsional stability for optimal callus formation
- Reusable implantation kit design requires no changes to established reamed insertion techniques
PANTHEON MEDICAL – Balanced Plating System
- Titanium-based plates intended for fixation of fractures, revision procedures, joint fusion, nonunions and reconstruction of small bone extremities
PANTHER ORTHOPEDICS – PUMA System
- Nitinol suture and PEEK anchor system used to treat ankle fractures, as well as ligament disruptions, soft tissue separation and hallux valgus of the foot
PRECIFIT MEDICAL – Kirschner Wires
- Stainless steel wire indicated for fixation of bone fractures, bone reconstruction and guide pins for insertion of other implants
- Relocated to U.S. from China to Research Triangle Park
RADICLE ORTHOPAEDICS – RASL Repair Kit/Bone Screw
- Designed to treat scapholunate ligament ruptures
- Screw allows for 15 to 22 degrees of toggle angle and freely rotates
SUBCHONDRAL SOLUTIONS – S4 Fixation Screw
- Fenestrated, cannulated system to treat osteochondral fracture
- Approach focuses on early biomechanical support for the entire bone/cartilage unit
TDM – Plate and Screw System
- Titanium flat and contoured plate and screw system intended for fracture fixation in the hand, wrist and small bones in the foot
- Korean company with more than two dozen trauma products in its portfolio
WISHBONE MEDICAL – K-Wire System
- Configured for pediatric elbow, long bone, extremity; small/medium/large kits, comprising two different sizes of wires, protective pin covers and instruments
WITTENSTEIN INTENS – Fitbone TAA Intramedullary Lengthening System
- Intended for lengthening of the femur and tibia via a nail with a telescoping system powered by an electromagnetic motor
- Other implants include custom-made lengthening systems
Carolyn LaWell is ORTHOWORLD’s Chief Content Officer. She can be reached by email.
Trauma remains one of the more attractive markets for orthopaedic device companies due to demographics, ripeness for innovation and an increased focus on lower extremities care, to name just three factors. These strengths have led companies of all sizes—established orthopaedic players and startups alike—to enter the trauma market. We think this...
Trauma remains one of the more attractive markets for orthopaedic device companies due to demographics, ripeness for innovation and an increased focus on lower extremities care, to name just three factors. These strengths have led companies of all sizes—established orthopaedic players and startups alike—to enter the trauma market. We think this dynamic will make trauma one of the more exciting markets to watch in coming years.
Nearly 200 companies worldwide sell implants and instruments for internal and external fracture repair. In 2017 the market reached $6.9 billion, +4.6% vs. 2016, according to our estimates published in THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT®.
As can be seen in Exhibits 1 and 2, DePuy Synthes is the dominant company in the segment, posting ~$2.5 billion in revenue in 2017, which accounted for 36% market share—a 16% lead over second place Stryker. DePuy Synthes and Stryker are well-positioned to maintain their hold on the market. Of interest is the amount of activity taking place among mid-tier and smaller trauma players vying for market share.
Exhibit 1: Trauma Sales Performance: Top 10 Players and All Others ($Millions)
| 2017 | 2016 | $ Change | % Change | |
| DePuy Synthes | $2,490.9 | $2,439.1 | $51.8 | 2.1% |
| Stryker | $1,378.1 | $1,265.8 | $112.3 | 8.9% |
| Zimmer Biomet | $560.2 | $536.3 | $23.9 | 4.5% |
| Smith & Nephew | $441.0 | $422.8 | $18.3 | 4.3% |
| Top-Tier Players Total | $4,870.2 | $4,663.5 | $206.7 | 4.4% |
| Wright Medical | $282.9 | $267.1 | $15.8 | 5.9% |
| Acumed | $200.0 | $189.4 | $10.6 | 5.6% |
| Aesculap | $123.2 | $117.9 | $5.3 | 4.5% |
| Osteomed | $109.7 | $104.5 | $5.2 | 5.0% |
| Orthofix | $103.2 | $102.7 | $0.5 | 0.5% |
| Medartis | $94.3 | $83.7 | $10.6 | 12.7% |
| Next-Tier Players Total | $913.3 | $865.3 | $48.0 | 5.6% |
| ~180 companies with revenue below $99MM | $1,136.5 | $1,086.0 | $50.5 | 4.7% |
| Total | $6,920.0 | $6,615.2 | $304.8 | 4.6% |
Exhibit 2: Trauma Market Share: Top 10 Players and All Others

In 2017, we noted 11 companies that received their first FDA 510(k) in trauma, and we identified an additional five companies in 1Q18. (See Exhibit 3 at the close of this article.) Most notably, spine company Globus Medical, which received clearance for products to treat shoulder, wrist and ankle fractures, and Germany-headquartered Wittenstein Intens, a multinational, multi-industry company that entered the U.S. market with its limb lengthening technology.
Additionally, in 2017, three notable acquisitions were made in the space. Arthrex purchased Sonoma Orthopedic Products, developer of wrist and clavicle implants. Medartis, prior to going public in 2018, acquired Mimedis, a Swiss startup that developed preoperative planning solutions. Osteomed bought OT Medical’s InstaFix shape memory staple systems.
We project the trauma market to grow in the high 4% range for each of the next five years, surpassing $8.7 billion by 2022.
The activity propelling this growth includes:
- A focus on lower extremity products. Many companies have made investments in this space, and we expect that the lifecycle of these products will play out over coming years, making this portion of the market worth watching.
- Prioritization of large companies. With patient demographics and expanding indications in mind, larger players will continue to grow their trauma portfolios to take advantage of cross-selling opportunities with other segments in which they play. Think DePuy Synthes and hip, and Wright Medical and extremities.
- Players of all sizes and from other segments will continue to make a concerted push in trauma. NuVasive and Globus Medical, traditionally spine companies, have entered the field. Arthrex, a traditionally arthroscopy/soft tissue repair company, has made acquisitions and received a handful of clearances in the last two years.
Exhibit 3: Companies Gaining First FDA 510(k) Clearances for Trauma Devices in 2017 and 1Q18
AKROS MEDICAL – Fibulink Syndesmosis Repair Kit
- Anchor to provide fixation between tibia and fibula after ankle syndesmosis
- Raised $610K in 4Q15 from private investors
ANJON HOLDINGS – Anjon Bermer Halo System
- Halo system intended to provide cervical spine immobilization for treatment of cervical trauma
- Bone anchorage elements include titanium threaded skull pins, aluminum crown and rod superstructure and polymer lined vest
EXOTOE – ExoToe Staple
- Intended for fracture, osteotomy fixation and joint arthrodesis of the foot in conjunction with k-wire fixation
- Provided in a sterile pack
EXSOMED – ArcPhix and ArrowPhix Small Bone Screw Systems
- Stainless steel compression screws for fixation of small bones, bone fragments, osteotomies (not soft tissue)
- Provided in a sterile, single-use kit
GLOBUS MEDICAL – Anthem Fracture System
- Made from titanium alloy, cobalt chromium, molybdenum alloy or stainless steel
- Comprises a Proximal Humerus Plating System for shoulder fractures, Clavicle Plating System, Comprehensive Distal Radius Fracture System, Proximal Tibia Plating, Comprehensive Ankle Fracture System and a Small Fragment Plating System
INTRAFUSE – Flex-Thread Clavicle Pin
- Stainless steel and PEEK, comprises a flexible threaded distal tip to engage the curved medial portion of the clavicle and a proximal cross screw to support stability and fixation to the lateral portion of the bone
- Portfolio company of incubator Surgical Frontiers that will focus on less invasive intramedullary fracture fixation of clavicle, radius, ulna and fibula
ORTHOVESTMENTS – Orbitum Bone Staple Implant X and VI
- Titanium-based implants ORBITUM X and VI Bone Staple indicated for hand and foot fractures
- ORTBITUM X and VI Implants intended for foot fractures, hallux valgus and primus varus treatment
ORTHOXEL – Apex Tibial Nail
- Reportedly offers the largest range of locking options of any intramedullary nail, and features a micromotion locking mode to support controlled axial movement with torsional stability for optimal callus formation
- Reusable implantation kit design requires no changes to established reamed insertion techniques
PANTHEON MEDICAL – Balanced Plating System
- Titanium-based plates intended for fixation of fractures, revision procedures, joint fusion, nonunions and reconstruction of small bone extremities
PANTHER ORTHOPEDICS – PUMA System
- Nitinol suture and PEEK anchor system used to treat ankle fractures, as well as ligament disruptions, soft tissue separation and hallux valgus of the foot
PRECIFIT MEDICAL – Kirschner Wires
- Stainless steel wire indicated for fixation of bone fractures, bone reconstruction and guide pins for insertion of other implants
- Relocated to U.S. from China to Research Triangle Park
RADICLE ORTHOPAEDICS – RASL Repair Kit/Bone Screw
- Designed to treat scapholunate ligament ruptures
- Screw allows for 15 to 22 degrees of toggle angle and freely rotates
SUBCHONDRAL SOLUTIONS – S4 Fixation Screw
- Fenestrated, cannulated system to treat osteochondral fracture
- Approach focuses on early biomechanical support for the entire bone/cartilage unit
TDM – Plate and Screw System
- Titanium flat and contoured plate and screw system intended for fracture fixation in the hand, wrist and small bones in the foot
- Korean company with more than two dozen trauma products in its portfolio
WISHBONE MEDICAL – K-Wire System
- Configured for pediatric elbow, long bone, extremity; small/medium/large kits, comprising two different sizes of wires, protective pin covers and instruments
WITTENSTEIN INTENS – Fitbone TAA Intramedullary Lengthening System
- Intended for lengthening of the femur and tibia via a nail with a telescoping system powered by an electromagnetic motor
- Other implants include custom-made lengthening systems
Carolyn LaWell is ORTHOWORLD’s Chief Content Officer. She can be reached by email.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
CL
Carolyn LaWell is ORTHOWORLD's Chief Content Officer. She joined ORTHOWORLD in 2012 to oversee its editorial and industry education. She previously served in editor roles at B2B magazines and newspapers.







