
 Copy to clipboard
Copy to clipboard 
Nearly 40% of spinal fusion patients suffer from chronic post-op pain and enter a years-long recovery journey that includes opioids, additional fusions, physical therapy and, eventually, neurostimulation to alleviate their aching back. The startup SynerFuse seeks to shorten that timeline with an integrated spinal fusion and neuromodulation concept that is intended to provide non-narcotic pain management.
“Pain is a significant problem in this country,” said SynerFuse CEO Justin Zenanko. “Over 50 million people suffer from chronic low back pain and over 1.1 million spinal procedures are performed each year. The problem that we’re in the pursuit of solving is worthy of investment to move this technology forward.”
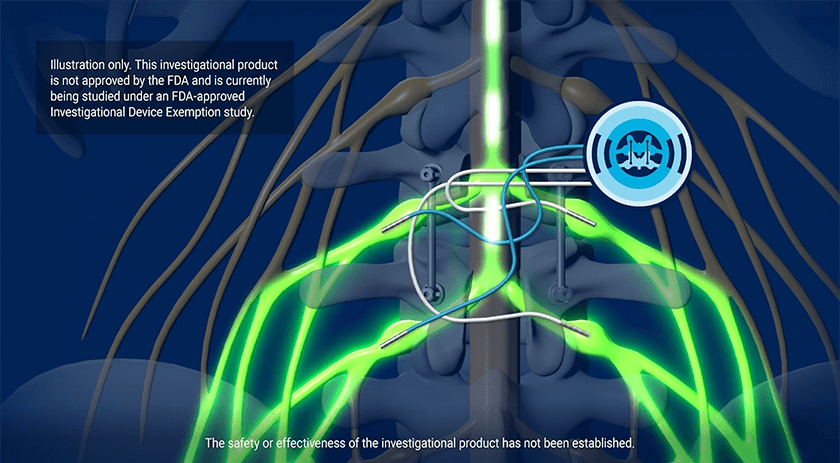
SynerFuse’s patented procedure, the Electric Transforaminal Lumbar Interbody Fusion (e-TLIFTM), uses the same line of sight to place the implant for a spinal fusion and the leads for stimulation on the nerves. “We’re targeting pain at the point of fusion, which has never been done before,” Mr. Zenanko said.
The Minnesota-based company has raised $23 million, of which $10 million is from Series A funding. SynerFuse seeks to raise an additional $6 million in its Series A. It has also secured more than 80 patents that combine neurostimulation with fusions, laminectomies and discectomies.
In October, the company will complete 12-month follow-ups on the 15 patients it enrolled in its FDA-approved IDE proof-of-concept study. It’s preparing to seek FDA approval for an IDE pivotal trial as the next step on its path to becoming an FDA-approved product.
We spoke with Mr. Zenanko about the company’s technology and next steps.
How do you describe your neuromodulation concept, and what makes it different from current treatment options?
Mr. Zenanko: We’re placing the implants for fusion and stimulation at the same time during one procedure. Our concept starts at the point of fusion and is turned on within the first 24 hours. That doesn’t occur anywhere else in today’s pain regime for stimulation.
The spine surgeon performs direct visual placement with a single incision. The NeuroFuse System is designed to eliminate the need for a neurostimulator pocket that is created with today’s stimulators for the treatment of pain. This device design should lead to greater adoption because the surgeon doesn’t need to go out of their field of work. You’re asking them to lay the leads directly on the nerve that they just freed during the decompression.
Also, we’re placing leads on the nerves, which means low energy is required. We took today’s off-the-shelf stimulator, a 32 CC (cubic centimeter) device and miniaturized it to nine CCs — it’s the size of a half dollar. Surgeons will not need to make another incision with a device that small.
A lot of patients who receive stimulation complain about pocket pain. We’re eliminating the pocket and decreasing the surgery time by implanting stimulation at the point of the fusion.
Are you targeting all fusions?
Mr. Zenanko: Based on the early data that we’re seeing, I believe every patient should have stimulation at the point of fusion as a hedge. To start, we’re targeting patients receiving first-time fusions at one or two levels who have been diagnosed with chronic pain in the lower back and legs.
What is the primary clinical problem you’re seeking to solve?
Mr. Zenanko: We want to improve patients’ pain in their legs and lower back, which will lead to an improvement in disability. This is a non-opioid intervention.
Today, you get a fusion and go home with opioids for pain relief. If you have a spasm or your pain flares up, the doctor prescribes more opioids. In our clinical trial, instead of prescribing opioids, the physicians changed the settings on the stimulator. There were several cases where opioids weren’t prescribed postoperatively.
Our early data shows that this is a path for eliminating narcotics from postoperative treatment plans. Opioids are a scourge in our society. This is the next advancement in fusion treatment.
Where are you with your IDE proof of concept study? What are the next steps?
Mr. Zenanko: We completed enrollment in October 2023, and the 15 patients will have completed their 12-month follow-up by this October.
We’re developing a proprietary device, the NeuroFuse system. It will be one of the smallest devices in the world for pain management. We are building the system based on the early data we’ve collected, energy usage requirements and components needed for a state-of-the-art device. We intend to conduct a national pivotal trial, which could include up to 20 sites and 150 first-time fusion patients.
We seek to build and complete the device in 2025. Based on early negotiations with FDA, we’re looking to start an IDE-approved pivotal trial in early 2026. We feel confident because we’re using real-world data to drive the design of our trial and novel device. It’s also informing our outreach to surgeons across the country.
What feedback have surgeons provided about the technology?
Mr. Zenanko: The feedback has been overwhelmingly positive, and surgeons want to participate in our next trial. Today, spine surgeons can perform a fusion procedure on patients with similar profiles and have vastly different outcomes for pain and disability. Fusions treat the spine’s mechanical problems — bone on bone, bone on nerve — but don’t treat the damaged nerves. Our technology intends to address both mechanical and neuropathic problems.
Think of the nerves as a garden hose. If you compress a garden hose over time, the hose won’t return to its normal shape. A similar situation occurs in the nerves of the back. If you can treat those nerves quickly enough through the mechanical piece, maybe the situation won’t become chronic. But I wouldn’t want to take that chance if I were a patient or a surgeon.
Why hasn’t this been done before?
Mr. Zenanko: There are barriers to overcome among industry, payors and older generations of surgeons.
A lot of the investments in spine are for 510(k) products. They’re small advancements. The industry is undergoing implant commoditization and developing and utilizing robotics for meaningful growth. They’re missing the big piece of the patient care puzzle, though, which is the nerves.
The medical profession has been divided into spine surgeons on one side and neuroscientists and pain physicians on the other. There isn’t a lot of collaboration. The reality is that only a spine surgeon can perform our eTLIF procedure. We’re trying to make the therapy of last resort — spine fusion — a better outcome for both patients and surgeons.
There are major companies that play in both spine and stimulation, but they’re more concerned about reimbursement and how they’re going to get paid. If the technology works and leads to long-term benefits, society will pay for it.
Finally, spine surgeons are the ones who can advance the technology. We’re a surgeon-led company. Our Chief Medical Officer, Dr. Rohan Lall, is a former investigator of our proof-of-concept trial and co-invented the e-TLIF procedure with Dr. Michael Park. We think about the surgeons and the patients. Overall, better procedures will lead to better outcomes that will lead to a better society for fusion patients who suffer from chronic low back and leg pain.
Nearly 40% of spinal fusion patients suffer from chronic post-op pain and enter a years-long recovery journey that includes opioids, additional fusions, physical therapy and, eventually, neurostimulation to alleviate their aching back. The startup SynerFuse seeks to shorten that timeline with an integrated spinal fusion and neuromodulation...
Nearly 40% of spinal fusion patients suffer from chronic post-op pain and enter a years-long recovery journey that includes opioids, additional fusions, physical therapy and, eventually, neurostimulation to alleviate their aching back. The startup SynerFuse seeks to shorten that timeline with an integrated spinal fusion and neuromodulation concept that is intended to provide non-narcotic pain management.
“Pain is a significant problem in this country,” said SynerFuse CEO Justin Zenanko. “Over 50 million people suffer from chronic low back pain and over 1.1 million spinal procedures are performed each year. The problem that we’re in the pursuit of solving is worthy of investment to move this technology forward.”
SynerFuse’s patented procedure, the Electric Transforaminal Lumbar Interbody Fusion (e-TLIFTM), uses the same line of sight to place the implant for a spinal fusion and the leads for stimulation on the nerves. “We’re targeting pain at the point of fusion, which has never been done before,” Mr. Zenanko said.
The Minnesota-based company has raised $23 million, of which $10 million is from Series A funding. SynerFuse seeks to raise an additional $6 million in its Series A. It has also secured more than 80 patents that combine neurostimulation with fusions, laminectomies and discectomies.
In October, the company will complete 12-month follow-ups on the 15 patients it enrolled in its FDA-approved IDE proof-of-concept study. It’s preparing to seek FDA approval for an IDE pivotal trial as the next step on its path to becoming an FDA-approved product.
We spoke with Mr. Zenanko about the company’s technology and next steps.
How do you describe your neuromodulation concept, and what makes it different from current treatment options?
Mr. Zenanko: We’re placing the implants for fusion and stimulation at the same time during one procedure. Our concept starts at the point of fusion and is turned on within the first 24 hours. That doesn’t occur anywhere else in today’s pain regime for stimulation.
The spine surgeon performs direct visual placement with a single incision. The NeuroFuse System is designed to eliminate the need for a neurostimulator pocket that is created with today’s stimulators for the treatment of pain. This device design should lead to greater adoption because the surgeon doesn’t need to go out of their field of work. You’re asking them to lay the leads directly on the nerve that they just freed during the decompression.
Also, we’re placing leads on the nerves, which means low energy is required. We took today’s off-the-shelf stimulator, a 32 CC (cubic centimeter) device and miniaturized it to nine CCs — it’s the size of a half dollar. Surgeons will not need to make another incision with a device that small.
A lot of patients who receive stimulation complain about pocket pain. We’re eliminating the pocket and decreasing the surgery time by implanting stimulation at the point of the fusion.
Are you targeting all fusions?
Mr. Zenanko: Based on the early data that we’re seeing, I believe every patient should have stimulation at the point of fusion as a hedge. To start, we’re targeting patients receiving first-time fusions at one or two levels who have been diagnosed with chronic pain in the lower back and legs.
What is the primary clinical problem you’re seeking to solve?
Mr. Zenanko: We want to improve patients’ pain in their legs and lower back, which will lead to an improvement in disability. This is a non-opioid intervention.
Today, you get a fusion and go home with opioids for pain relief. If you have a spasm or your pain flares up, the doctor prescribes more opioids. In our clinical trial, instead of prescribing opioids, the physicians changed the settings on the stimulator. There were several cases where opioids weren’t prescribed postoperatively.
Our early data shows that this is a path for eliminating narcotics from postoperative treatment plans. Opioids are a scourge in our society. This is the next advancement in fusion treatment.
Where are you with your IDE proof of concept study? What are the next steps?
Mr. Zenanko: We completed enrollment in October 2023, and the 15 patients will have completed their 12-month follow-up by this October.
We’re developing a proprietary device, the NeuroFuse system. It will be one of the smallest devices in the world for pain management. We are building the system based on the early data we’ve collected, energy usage requirements and components needed for a state-of-the-art device. We intend to conduct a national pivotal trial, which could include up to 20 sites and 150 first-time fusion patients.
We seek to build and complete the device in 2025. Based on early negotiations with FDA, we’re looking to start an IDE-approved pivotal trial in early 2026. We feel confident because we’re using real-world data to drive the design of our trial and novel device. It’s also informing our outreach to surgeons across the country.
What feedback have surgeons provided about the technology?
Mr. Zenanko: The feedback has been overwhelmingly positive, and surgeons want to participate in our next trial. Today, spine surgeons can perform a fusion procedure on patients with similar profiles and have vastly different outcomes for pain and disability. Fusions treat the spine’s mechanical problems — bone on bone, bone on nerve — but don’t treat the damaged nerves. Our technology intends to address both mechanical and neuropathic problems.
Think of the nerves as a garden hose. If you compress a garden hose over time, the hose won’t return to its normal shape. A similar situation occurs in the nerves of the back. If you can treat those nerves quickly enough through the mechanical piece, maybe the situation won’t become chronic. But I wouldn’t want to take that chance if I were a patient or a surgeon.
Why hasn’t this been done before?
Mr. Zenanko: There are barriers to overcome among industry, payors and older generations of surgeons.
A lot of the investments in spine are for 510(k) products. They’re small advancements. The industry is undergoing implant commoditization and developing and utilizing robotics for meaningful growth. They’re missing the big piece of the patient care puzzle, though, which is the nerves.
The medical profession has been divided into spine surgeons on one side and neuroscientists and pain physicians on the other. There isn’t a lot of collaboration. The reality is that only a spine surgeon can perform our eTLIF procedure. We’re trying to make the therapy of last resort — spine fusion — a better outcome for both patients and surgeons.
There are major companies that play in both spine and stimulation, but they’re more concerned about reimbursement and how they’re going to get paid. If the technology works and leads to long-term benefits, society will pay for it.
Finally, spine surgeons are the ones who can advance the technology. We’re a surgeon-led company. Our Chief Medical Officer, Dr. Rohan Lall, is a former investigator of our proof-of-concept trial and co-invented the e-TLIF procedure with Dr. Michael Park. We think about the surgeons and the patients. Overall, better procedures will lead to better outcomes that will lead to a better society for fusion patients who suffer from chronic low back and leg pain.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
CL
Carolyn LaWell is ORTHOWORLD's Chief Content Officer. She joined ORTHOWORLD in 2012 to oversee its editorial and industry education. She previously served in editor roles at B2B magazines and newspapers.







