
 Copy to clipboard
Copy to clipboard 
SurGenTec received additional FDA 510(k) clearance for OsteoFlo HydroFiber synthetic bone graft, marking another key regulatory milestone. This expansion of indications now includes use as a bone void filler for the treatment of tumors, cysts, trauma and osteomyelitis.
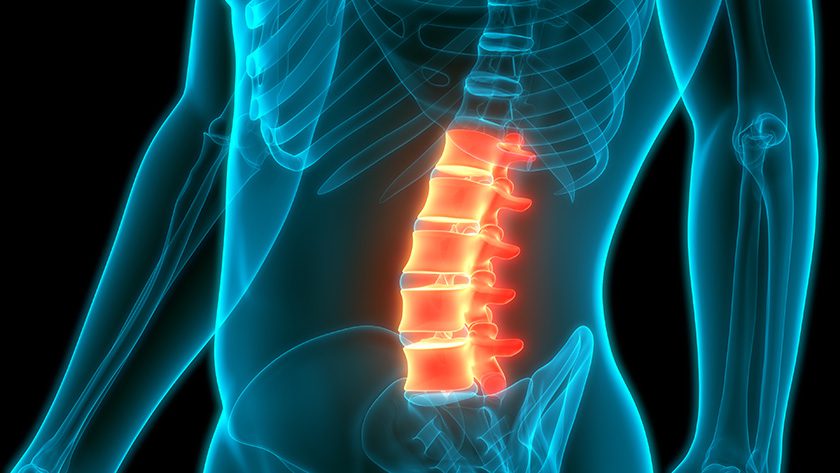
This latest addition complements SurGenTec’s previously clearance, which is recognized for its equivalence to autograft in spine surgery and is versatile enough for use at any spinal level, including for posterolateral fusions, disc spaces or interbody fusion cages (FDA cleared).
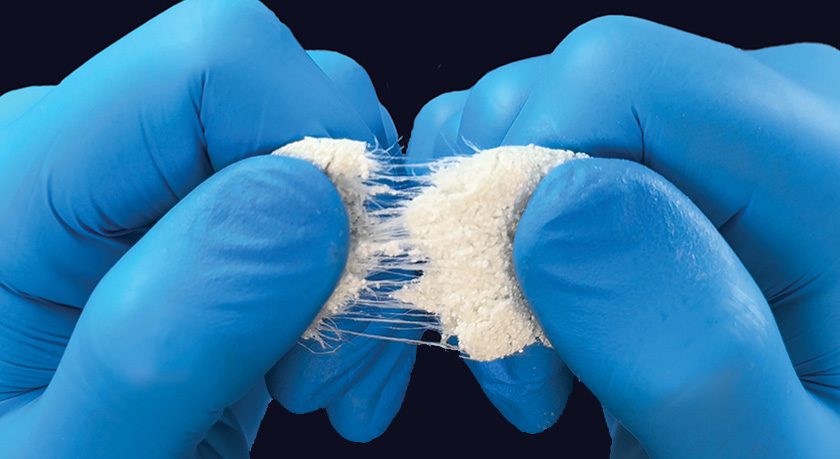
OsteoFlo HydroFiber features advanced Web Interlace Technology, which suspends particles within its fibers to effectively prevent graft migration, ensuring optimal cohesiveness and flowability. The intricate hydrophilic bonds and proprietary fibers allow for the wicking of saline, blood, or bone marrow aspirate, creating a robust platform to support the healing process.
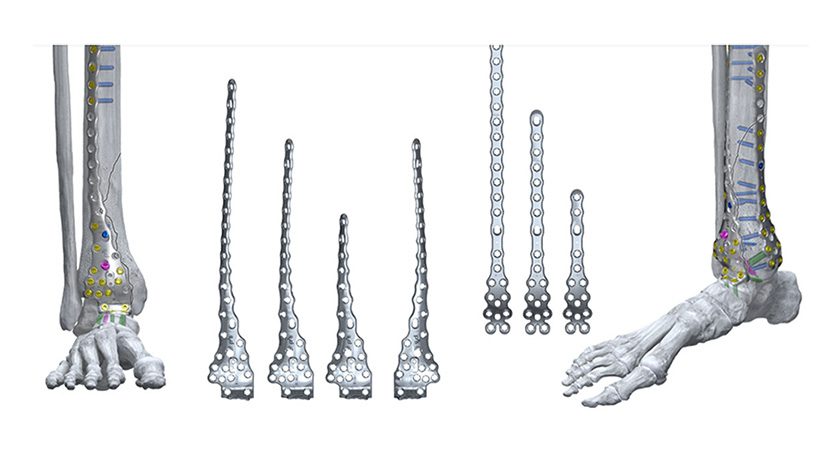
The product’s formulation guarantees optimal handling and resists migration under irrigation, permitting seamless application across various surgical settings, including spine, orthopedics, and foot/ankle procedures. Additionally, it can be utilized with the GraftGun, SurGenTec’s flagship delivery device, to efficiently backfill interbody cages and tight spaces—a significant enhancement over traditional funnel methods.
“Receiving this FDA clearance for OsteoFlo HydroFiber is a major milestone for SurGenTec,” stated Travis Greenhalgh, CEO and founder of SurGenTec. “Our team has worked diligently in product development and testing to extend our capabilities and support more patients undergoing complex surgeries. Treating tumors and osteomyelitis presents significant challenges with limited options available. Our technology offers a powerful, autograft-equivalent solution, providing optimal clinical outcomes without the need for autograft harvesting. This allows physicians to avoid the invasive procedure and risks associated with autograft while still delivering exceptional results, positioning our product as the superior choice over allograft.”
Source: SurGenTec
SurGenTec received additional FDA 510(k) clearance for OsteoFlo HydroFiber synthetic bone graft, marking another key regulatory milestone. This expansion of indications now includes use as a bone void filler for the treatment of tumors, cysts, trauma and osteomyelitis.
This latest addition complements SurGenTec’s previously clearance,...
SurGenTec received additional FDA 510(k) clearance for OsteoFlo HydroFiber synthetic bone graft, marking another key regulatory milestone. This expansion of indications now includes use as a bone void filler for the treatment of tumors, cysts, trauma and osteomyelitis.
This latest addition complements SurGenTec’s previously clearance, which is recognized for its equivalence to autograft in spine surgery and is versatile enough for use at any spinal level, including for posterolateral fusions, disc spaces or interbody fusion cages (FDA cleared).
OsteoFlo HydroFiber features advanced Web Interlace Technology, which suspends particles within its fibers to effectively prevent graft migration, ensuring optimal cohesiveness and flowability. The intricate hydrophilic bonds and proprietary fibers allow for the wicking of saline, blood, or bone marrow aspirate, creating a robust platform to support the healing process.
The product’s formulation guarantees optimal handling and resists migration under irrigation, permitting seamless application across various surgical settings, including spine, orthopedics, and foot/ankle procedures. Additionally, it can be utilized with the GraftGun, SurGenTec’s flagship delivery device, to efficiently backfill interbody cages and tight spaces—a significant enhancement over traditional funnel methods.
“Receiving this FDA clearance for OsteoFlo HydroFiber is a major milestone for SurGenTec,” stated Travis Greenhalgh, CEO and founder of SurGenTec. “Our team has worked diligently in product development and testing to extend our capabilities and support more patients undergoing complex surgeries. Treating tumors and osteomyelitis presents significant challenges with limited options available. Our technology offers a powerful, autograft-equivalent solution, providing optimal clinical outcomes without the need for autograft harvesting. This allows physicians to avoid the invasive procedure and risks associated with autograft while still delivering exceptional results, positioning our product as the superior choice over allograft.”
Source: SurGenTec

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.