
 Copy to clipboard
Copy to clipboard 
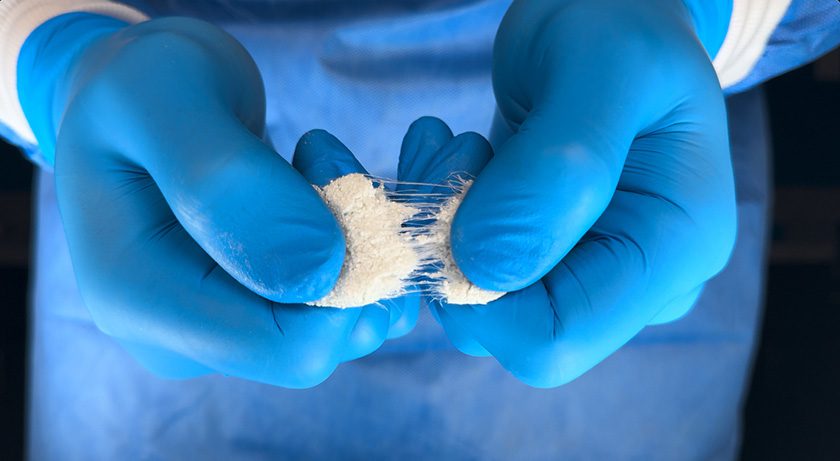
SurGenTec received FDA 510(k) clearance to market OsteoFlo HydroFiber bone graft technology, a stand-alone solution equivalent to autograft for spine surgeries, including use in interbody fusion cages, disc spaces and posterolateral fusions.
OsteoFlo HydroFiber is slated for launch in early 2025.
The product leverages Web Interlace Technology, suspending particles within its fibers to effectively prevent graft migration while ensuring optimal cohesiveness and flowability. The product employs hydrophilic bonds and proprietary fibers that absorb saline, blood or bone marrow aspirate while establishing a platform to support the healing process.
The product can also be used in conjunction with the Graftgun, SurGenTec’s flagship delivery device, for efficiently backfilling interbody cages—an alternative to traditional funnel methods.
“This product is set to revolutionize bone graft technology,” said Travis Greenhalgh, CEO and founder of SurGenTec. “OsteoFlo HydroFiber provides a novel, customizable solution for surgeons, tailored to both anatomy and procedure type. Physicians are familiar with the handling characteristics of fiber-based allograft products, and we are proud to introduce a synthetic option designed to accelerate healing without the risk of human allograft tissue.”
Source: SurGenTec
SurGenTec received FDA 510(k) clearance to market OsteoFlo HydroFiber bone graft technology, a stand-alone solution equivalent to autograft for spine surgeries, including use in interbody fusion cages, disc spaces and posterolateral fusions.
OsteoFlo HydroFiber is slated for launch in early 2025.
The product leverages Web Interlace...
SurGenTec received FDA 510(k) clearance to market OsteoFlo HydroFiber bone graft technology, a stand-alone solution equivalent to autograft for spine surgeries, including use in interbody fusion cages, disc spaces and posterolateral fusions.
OsteoFlo HydroFiber is slated for launch in early 2025.
The product leverages Web Interlace Technology, suspending particles within its fibers to effectively prevent graft migration while ensuring optimal cohesiveness and flowability. The product employs hydrophilic bonds and proprietary fibers that absorb saline, blood or bone marrow aspirate while establishing a platform to support the healing process.
The product can also be used in conjunction with the Graftgun, SurGenTec’s flagship delivery device, for efficiently backfilling interbody cages—an alternative to traditional funnel methods.
“This product is set to revolutionize bone graft technology,” said Travis Greenhalgh, CEO and founder of SurGenTec. “OsteoFlo HydroFiber provides a novel, customizable solution for surgeons, tailored to both anatomy and procedure type. Physicians are familiar with the handling characteristics of fiber-based allograft products, and we are proud to introduce a synthetic option designed to accelerate healing without the risk of human allograft tissue.”
Source: SurGenTec

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







