
 Copy to clipboard
Copy to clipboard 
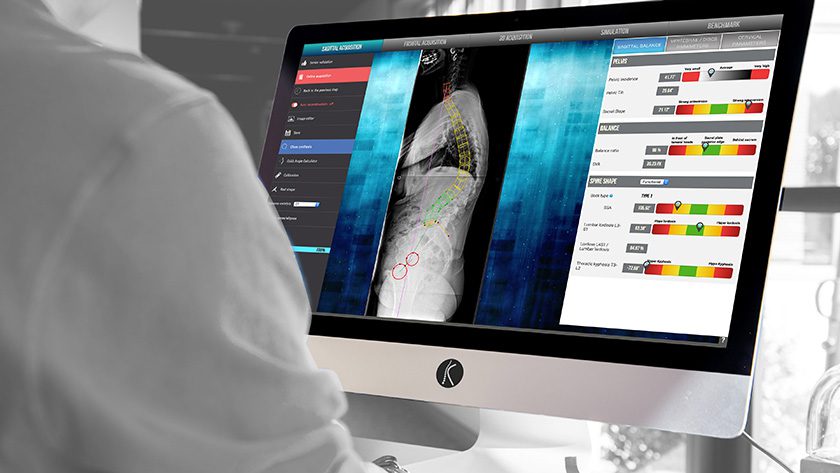
SMAIO (Software, Machines and Adaptative Implants in Orthopaedics) received FDA 510(k) clearance to market a customized surgery planning software co-developed with NuVasive.
This customized surgery planning software is based on SMAIO’s previously released vertebral realignment planning technology, Balance Analyzer 3D.
The achievement of this first milestone triggers a $3 million payment by NuVasive to SMAIO. As part of the previously announced partnership, an additional milestone payment will be triggered after further integration of the Balance Analyzer 3D into the NuVasive platform, as well as additional technical capabilities.
Philippe Roussouly, CEO of SMAIO, said, “This 510(k) clearance represents a major step towards the commercialization of our joint planning platform and innovative services to NuVasive customers. We believe that with our technology, surgeons will soon benefit from our cutting-edge solution for planning and performing spine surgery, considering individual morphological specificities and ultimately, for improving patient outcomes.”
Source: SMAIO
SMAIO (Software, Machines and Adaptative Implants in Orthopaedics) received FDA 510(k) clearance to market a customized surgery planning software co-developed with NuVasive.
This customized surgery planning software is based on SMAIO’s previously released vertebral realignment planning technology, Balance Analyzer 3D.
The achievement of...
SMAIO (Software, Machines and Adaptative Implants in Orthopaedics) received FDA 510(k) clearance to market a customized surgery planning software co-developed with NuVasive.
This customized surgery planning software is based on SMAIO’s previously released vertebral realignment planning technology, Balance Analyzer 3D.
The achievement of this first milestone triggers a $3 million payment by NuVasive to SMAIO. As part of the previously announced partnership, an additional milestone payment will be triggered after further integration of the Balance Analyzer 3D into the NuVasive platform, as well as additional technical capabilities.
Philippe Roussouly, CEO of SMAIO, said, “This 510(k) clearance represents a major step towards the commercialization of our joint planning platform and innovative services to NuVasive customers. We believe that with our technology, surgeons will soon benefit from our cutting-edge solution for planning and performing spine surgery, considering individual morphological specificities and ultimately, for improving patient outcomes.”
Source: SMAIO

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







