
 Copy to clipboard
Copy to clipboard 
SMAIO (Software, Machines and Adaptative Implants in Orthopaedics) received FDA 510(k) clearance for its Balance Analyzer 3D surgery planning software.
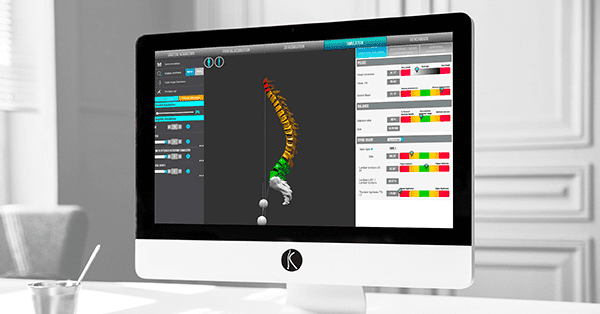
Balance Analyzer 3D is spinal realignment planning software that uses medical imaging of the patient’s spine. Since receiving approval under the CE Mark in 2014, it has been an integral part of the i-plan surgical planning solution (shown above) marketed by SMAIO within its comprehensive i-kontrol platform that allows surgeons to treat spinal pathologies. i-kontrol incorporates planning, implants and related services.
Obtaining FDA 510(k) clearance is a key element of a partnership and licensing agreement signed in 1Q22 between SMAIO and NuVasive to further develop SMAIO’s surgical planning solutions and to support the innovation pipeline and commercialization efforts.
As part of this collaboration, NuVasive pledged to invest a total of $10 million in SMAIO, of which $5 million was already invested within the framework of SMAIO’s IPO in early April. The balance will consist of milestone payments made when SMAIO receives FDA 510(k) clearance for two software solutions interfacing with NuVasive’s technological platforms.
NuVasive has exclusivity regarding the planning tool development partnerships implemented by SMAIO for a three-year period from the date on which the second software solution is approved.
Further, SMAIO will put in place an image analysis and planning assistance service for NuVasive’s clients, which will be billed and will thus generate recurring payments proportional to the number of analyses performed.
Philippe Roussouly, CEO of SMAIO, said, “This 510(k) clearance for the current version of our Balance Analyzer 3D planning software represents a major step in our American market penetration strategy that will be based, in the coming years, on our partnership with NuVasive. It lays the foundations for the co-developments the two companies have agreed to carry out over the next two years, and in the very near term will enable us both to initiate a certain number of technological and scientific collaborations with centers of excellence in the United States.”
Source: SMAIO
SMAIO (Software, Machines and Adaptative Implants in Orthopaedics) received FDA 510(k) clearance for its Balance Analyzer 3D surgery planning software.
Balance Analyzer 3D is spinal realignment planning software that uses medical imaging of the patient’s spine. Since receiving approval under the CE Mark in 2014, it has been an integral part of...
SMAIO (Software, Machines and Adaptative Implants in Orthopaedics) received FDA 510(k) clearance for its Balance Analyzer 3D surgery planning software.
Balance Analyzer 3D is spinal realignment planning software that uses medical imaging of the patient’s spine. Since receiving approval under the CE Mark in 2014, it has been an integral part of the i-plan surgical planning solution (shown above) marketed by SMAIO within its comprehensive i-kontrol platform that allows surgeons to treat spinal pathologies. i-kontrol incorporates planning, implants and related services.
Obtaining FDA 510(k) clearance is a key element of a partnership and licensing agreement signed in 1Q22 between SMAIO and NuVasive to further develop SMAIO’s surgical planning solutions and to support the innovation pipeline and commercialization efforts.
As part of this collaboration, NuVasive pledged to invest a total of $10 million in SMAIO, of which $5 million was already invested within the framework of SMAIO’s IPO in early April. The balance will consist of milestone payments made when SMAIO receives FDA 510(k) clearance for two software solutions interfacing with NuVasive’s technological platforms.
NuVasive has exclusivity regarding the planning tool development partnerships implemented by SMAIO for a three-year period from the date on which the second software solution is approved.
Further, SMAIO will put in place an image analysis and planning assistance service for NuVasive’s clients, which will be billed and will thus generate recurring payments proportional to the number of analyses performed.
Philippe Roussouly, CEO of SMAIO, said, “This 510(k) clearance for the current version of our Balance Analyzer 3D planning software represents a major step in our American market penetration strategy that will be based, in the coming years, on our partnership with NuVasive. It lays the foundations for the co-developments the two companies have agreed to carry out over the next two years, and in the very near term will enable us both to initiate a certain number of technological and scientific collaborations with centers of excellence in the United States.”
Source: SMAIO

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







