
 Copy to clipboard
Copy to clipboard 
The first U.S. surgical procedure was successfully performed using Premia Spine’s TOPS System, a motion preservation spinal device, immediately following its approval by FDA.
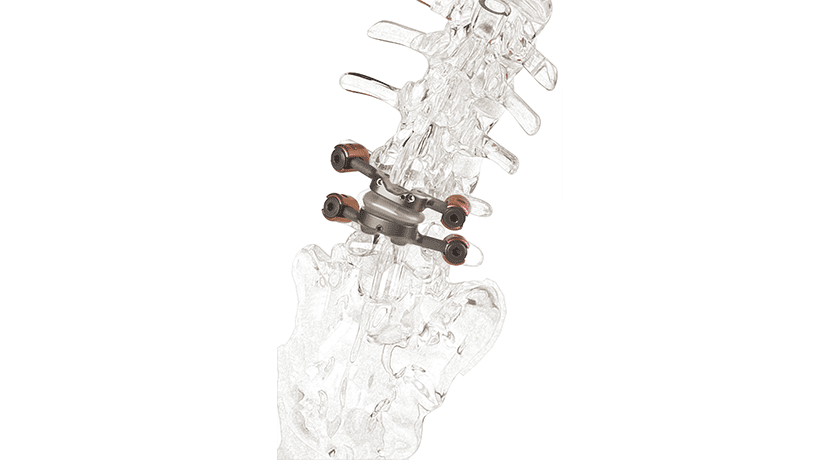
The TOPS System is an innovative solution designed to treat spinal stenosis and spondylolisthesis. This FDA approval follows extensive clinical trials that demonstrated the system’s safety and effectiveness in alleviating back pain and improving physical function.
The TOPS System, the first and only lumbar spine facet joint replacement device, provides a beacon of hope for the over 100 million individuals globally grappling with the debilitating effects of lumbar spinal stenosis and degenerative spondylolisthesis. As the only technology of its kind designed to preserve mobility post-decompression, TOPS offers an alternative to the 350,000 patients annually who traditionally undergo lumbar spinal fusion.
“Spinal stenosis and spondylolisthesis patients now have access to a lumbar motion device that is superior to fusion, according to its FDA label,” said Ron Sacher, CEO of Premia Spine. “We are thrilled to bring the TOPS System to patients who seek a non-fusion solution for their leg and back pain. We are very grateful to all the surgeons who made it possible to bring our facet replacement product to the U.S. market, and we are particularly fortunate to have Dr. Jared Ament perform the first commercial TOPS procedure.”
“This milestone is more than just a testament to our technology—it’s a testament to the hard work and dedication of our entire team,” added Peter Wehrly, President of Premia Spine. “We’re excited to see what the future holds as we continue to drive advancements in spine care.”
Source: Premia Spine
The first U.S. surgical procedure was successfully performed using Premia Spine’s TOPS System, a motion preservation spinal device, immediately following its approval by FDA.
The TOPS System is an innovative solution designed to treat spinal stenosis and spondylolisthesis. This FDA approval follows extensive clinical trials that...
The first U.S. surgical procedure was successfully performed using Premia Spine’s TOPS System, a motion preservation spinal device, immediately following its approval by FDA.
The TOPS System is an innovative solution designed to treat spinal stenosis and spondylolisthesis. This FDA approval follows extensive clinical trials that demonstrated the system’s safety and effectiveness in alleviating back pain and improving physical function.
The TOPS System, the first and only lumbar spine facet joint replacement device, provides a beacon of hope for the over 100 million individuals globally grappling with the debilitating effects of lumbar spinal stenosis and degenerative spondylolisthesis. As the only technology of its kind designed to preserve mobility post-decompression, TOPS offers an alternative to the 350,000 patients annually who traditionally undergo lumbar spinal fusion.
“Spinal stenosis and spondylolisthesis patients now have access to a lumbar motion device that is superior to fusion, according to its FDA label,” said Ron Sacher, CEO of Premia Spine. “We are thrilled to bring the TOPS System to patients who seek a non-fusion solution for their leg and back pain. We are very grateful to all the surgeons who made it possible to bring our facet replacement product to the U.S. market, and we are particularly fortunate to have Dr. Jared Ament perform the first commercial TOPS procedure.”
“This milestone is more than just a testament to our technology—it’s a testament to the hard work and dedication of our entire team,” added Peter Wehrly, President of Premia Spine. “We’re excited to see what the future holds as we continue to drive advancements in spine care.”
Source: Premia Spine

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







