
 Copy to clipboard
Copy to clipboard 
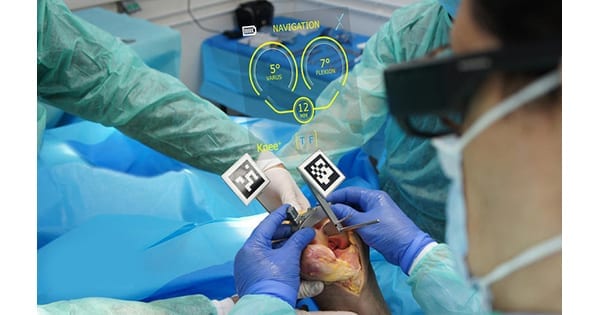
Pixee Medical entered into an agreement with United Orthopedic Corporation (UOC) for distribution of Knee+, the first worldwide augmented reality (AR) navigation system for total knee replacement. Under this agreement, UOC will serve as a non-exclusive distributor for selected countries in Europe.
Knee+ technology combines proprietary computer vision and deep learning algorithms, and can be used with off-the-shelf AR glasses to track instruments and implants during a procedure. The navigation software installed in smart glasses is combined with reduced-size MIS instrumentation equipped with markers that can be sterilized in an autoclave.
The first system will be installed in France before the end of October. After limited release in Europe, Pixee Medical will expand quickly in Australia and in the U.S. following FDA 510(k) market clearance.
Knee+ was granted approval under the CE Mark earlier this year.
Pixee Medical entered into an agreement with United Orthopedic Corporation (UOC) for distribution of Knee+, the first worldwide augmented reality (AR) navigation system for total knee replacement. Under this agreement, UOC will serve as a non-exclusive distributor for selected countries in Europe.
Knee+ technology combines proprietary computer...
Pixee Medical entered into an agreement with United Orthopedic Corporation (UOC) for distribution of Knee+, the first worldwide augmented reality (AR) navigation system for total knee replacement. Under this agreement, UOC will serve as a non-exclusive distributor for selected countries in Europe.
Knee+ technology combines proprietary computer vision and deep learning algorithms, and can be used with off-the-shelf AR glasses to track instruments and implants during a procedure. The navigation software installed in smart glasses is combined with reduced-size MIS instrumentation equipped with markers that can be sterilized in an autoclave.
The first system will be installed in France before the end of October. After limited release in Europe, Pixee Medical will expand quickly in Australia and in the U.S. following FDA 510(k) market clearance.
Knee+ was granted approval under the CE Mark earlier this year.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







