
 Copy to clipboard
Copy to clipboard 
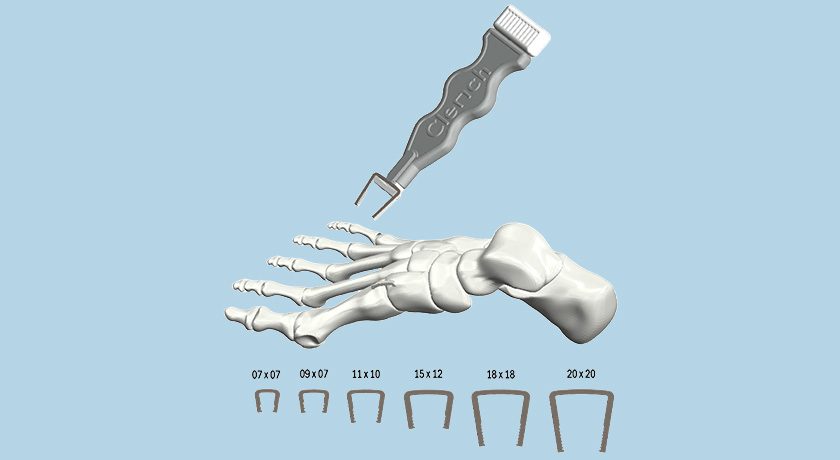
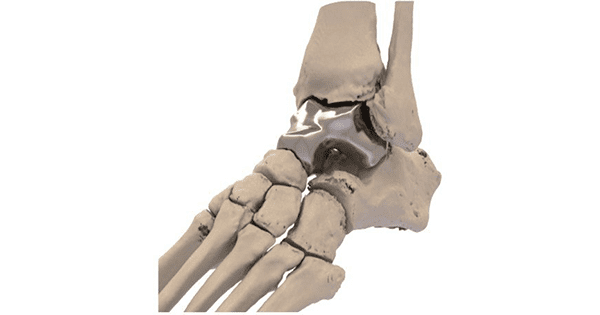
Paragon 28 acquired the product lines of Additive Orthopaedics, including the Additive 3D-printed Patient Specific Talus Spacer.
Earlier this year, FDA approved the spacer as the first and only patient-specific total talus replacement implant authorized for use in the U.S. as a humanitarian use device and provides a joint-sparing alternative to traditional ankle fusion.
Also acquired was Additive’s internally developed, proprietary pre-operative surgical planning application, a fully integrated cloud-based communication tool that aligns surgeons and engineers to design patient specific surgical plans and implants.
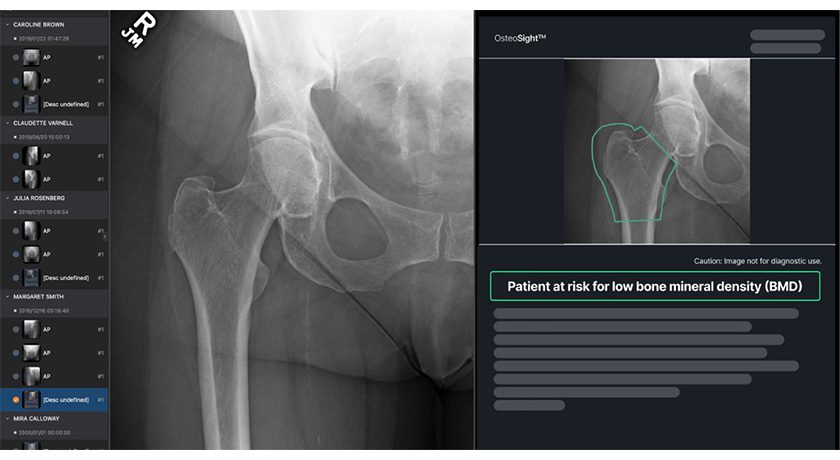
“The addition of the Additive Orthopaedics product portfolio and surgical planning capabilities provides Paragon 28 customers exclusive access to the only FDA-approved patient specific total talus replacement implant,” said Albert DaCosta, Co-Founder and Chief Executive Officer of Paragon 28. “The Additive acquisition also significantly accelerates the Company’s strategy to leverage smart tools, artificial intelligence and advanced technology to improve patient outcomes.”
Paragon 28 acquired the product lines of Additive Orthopaedics, including the Additive 3D-printed Patient Specific Talus Spacer.
Earlier this year, FDA approved the spacer as the first and only patient-specific total talus replacement implant authorized for use in the U.S. as a humanitarian use device and provides a joint-sparing...
Paragon 28 acquired the product lines of Additive Orthopaedics, including the Additive 3D-printed Patient Specific Talus Spacer.
Earlier this year, FDA approved the spacer as the first and only patient-specific total talus replacement implant authorized for use in the U.S. as a humanitarian use device and provides a joint-sparing alternative to traditional ankle fusion.
Also acquired was Additive’s internally developed, proprietary pre-operative surgical planning application, a fully integrated cloud-based communication tool that aligns surgeons and engineers to design patient specific surgical plans and implants.
“The addition of the Additive Orthopaedics product portfolio and surgical planning capabilities provides Paragon 28 customers exclusive access to the only FDA-approved patient specific total talus replacement implant,” said Albert DaCosta, Co-Founder and Chief Executive Officer of Paragon 28. “The Additive acquisition also significantly accelerates the Company’s strategy to leverage smart tools, artificial intelligence and advanced technology to improve patient outcomes.”

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.