
 Copy to clipboard
Copy to clipboard 
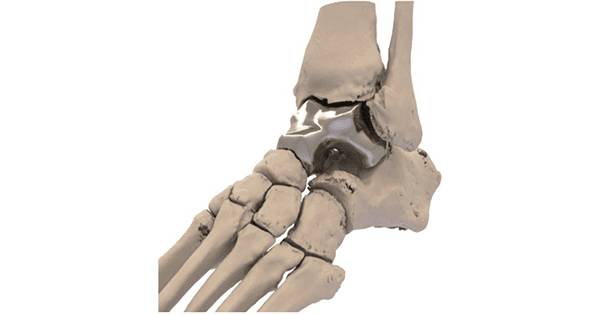
Additive Orthopaedics received an approval order from FDA for its Humanitarian Device Exemption (HDE) application for the Patient Specific Talus Spacer for treatment of avascular necrosis of the talus. This is the first patient specific total talus replacement implant approved by FDA.
Under the HDE, the Patient Specific Talus Spacer will be made available as a humanitarian use device. Commercial U.S. launch will commence immediately.
The Patient Specific Talus Spacer is an additively manufactured device that allows a patient to regain motion and reduce pain until the time a fusion may become necessary.
Greg Kowalczyk, President of Additive Orthopaedics, said, “Avascular necrosis of the talus is extremely painful and debilitating for these patients. Surgical treatment options are below-the-knee amputation or joint fusion, which results in loss of motion of the ankle and can have poor outcomes. The Patient Specific Talus Spacer is another example of how 3D-printed devices can improve the standard of care.”
Additive Orthopaedics received an approval order from FDA for its Humanitarian Device Exemption (HDE) application for the Patient Specific Talus Spacer for treatment of avascular necrosis of the talus. This is the first patient specific total talus replacement implant approved by FDA.
Under the HDE, the Patient Specific Talus Spacer will...
Additive Orthopaedics received an approval order from FDA for its Humanitarian Device Exemption (HDE) application for the Patient Specific Talus Spacer for treatment of avascular necrosis of the talus. This is the first patient specific total talus replacement implant approved by FDA.
Under the HDE, the Patient Specific Talus Spacer will be made available as a humanitarian use device. Commercial U.S. launch will commence immediately.
The Patient Specific Talus Spacer is an additively manufactured device that allows a patient to regain motion and reduce pain until the time a fusion may become necessary.
Greg Kowalczyk, President of Additive Orthopaedics, said, “Avascular necrosis of the talus is extremely painful and debilitating for these patients. Surgical treatment options are below-the-knee amputation or joint fusion, which results in loss of motion of the ankle and can have poor outcomes. The Patient Specific Talus Spacer is another example of how 3D-printed devices can improve the standard of care.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







