
 Copy to clipboard
Copy to clipboard 
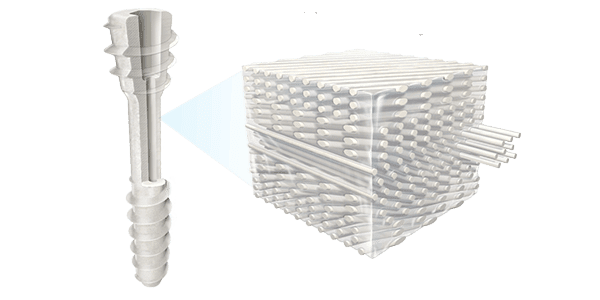
OSSIO commenced U.S. launch of OSSIOfiber® Compression Screws for maintenance of alignment and fixation of bone fractures, comminuted fractures, fragments, osteotomies, arthrodesis and bone grafts of the upper extremity, fibula, knee, ankle and foot in the presence of appropriate brace and/or immobilization.
First commercial cases have been successfully conducted during limited launch. Full commercialization is slated for April 2021.
The portfolio received FDA 510(k) market clearance in 2020. The initial product offering is applicable for use in the treatment of multiple lower and upper extremity injuries.
Comprising proprietary OSSIOfiber Intelligent Bone Regeneration Technology, the screws are engineered to achieve the optimal balance of flexural, torque, axial and shear strength.
OSSIO plans to expand its compression screw portfolio in varying diameters, lengths and geometry to address additional extremity procedures as well as trauma cases in the near future.
The company has also closed a $20 million financing deal to support aggressive growth plans. OSSIO intends to pursue multiple applications in the distal extremity, trauma, sports, reconstruction, pediatrics and spine segments.
OSSIO commenced U.S. launch of OSSIOfiber® Compression Screws for maintenance of alignment and fixation of bone fractures, comminuted fractures, fragments, osteotomies, arthrodesis and bone grafts of the upper extremity, fibula, knee, ankle and foot in the presence of appropriate brace and/or immobilization.
First commercial cases have...
OSSIO commenced U.S. launch of OSSIOfiber® Compression Screws for maintenance of alignment and fixation of bone fractures, comminuted fractures, fragments, osteotomies, arthrodesis and bone grafts of the upper extremity, fibula, knee, ankle and foot in the presence of appropriate brace and/or immobilization.
First commercial cases have been successfully conducted during limited launch. Full commercialization is slated for April 2021.
The portfolio received FDA 510(k) market clearance in 2020. The initial product offering is applicable for use in the treatment of multiple lower and upper extremity injuries.
Comprising proprietary OSSIOfiber Intelligent Bone Regeneration Technology, the screws are engineered to achieve the optimal balance of flexural, torque, axial and shear strength.
OSSIO plans to expand its compression screw portfolio in varying diameters, lengths and geometry to address additional extremity procedures as well as trauma cases in the near future.
The company has also closed a $20 million financing deal to support aggressive growth plans. OSSIO intends to pursue multiple applications in the distal extremity, trauma, sports, reconstruction, pediatrics and spine segments.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







