
 Copy to clipboard
Copy to clipboard 
OSSIO received FDA 510(k) clearance to market its OSSIOfiber® Compression Screw, indicated for maintenance of alignment and fixation of bone fractures, comminuted fractures, fragments, osteotomies, arthrodesis and bone grafts of the upper extremity, fibula, knee, ankle and foot in the presence of appropriate brace and/or immobilization.
OSSIO’s Compression Screw Portfolio will initially comprise a 4.0mm-diameter cannulated, headless, partially threaded compression screw in lengths ranging from 26mm to 60mm. Commercial launch will begin in early 2021.
OSSIOfiber uses bio-integrative material to provide stability and secure bone fixation while leaving no permanent hardware behind.
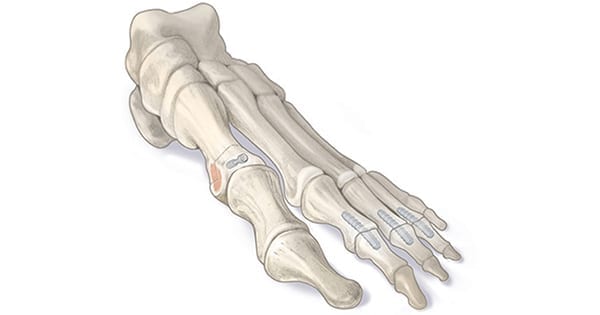
The company recently reached its 1,000th procedural milestone utilizing OSSIOfiber implants, including both OSSIOfiber Hammertoe Fixation (shown above) and the OSSIOfiber Trimmable Fixation Nail. Since 2019, U.S. surgeons have used the implants in a range of orthopedic procedures – including forefoot, midfoot, hindfoot and hand/wrist – with high satisfaction rates and no reported revision cases to date.
The bio-integrative implant is reportedly the only compression screw on the market that combines the necessary strength for bone fixation with the ability to fully integrate into the surrounding anatomy without adverse foreign body reactions and stress shielding, and avoids potential patient discomfort or implant related complications that are often associated with permanent metal hardware.
Brian Verrier, CEO, said, “The achievement of our 1,000th implant milestone, along with the recent FDA clearance for our Compression Screw Portfolio, further showcase our commitment to changing a 100-year-old standard-of-care in orthopedic fixation, transforming the patient experience and improving the overall healthcare economics of orthopedics.”
The company intends to pursue multiple applications for OSSIOfiber in the distal extremity, trauma, sports, reconstruction, pediatrics and spine segments.
OSSIO received FDA 510(k) clearance to market its OSSIOfiber® Compression Screw, indicated for maintenance of alignment and fixation of bone fractures, comminuted fractures, fragments, osteotomies, arthrodesis and bone grafts of the upper extremity, fibula, knee, ankle and foot in the presence of appropriate brace and/or immobilization.
...
OSSIO received FDA 510(k) clearance to market its OSSIOfiber® Compression Screw, indicated for maintenance of alignment and fixation of bone fractures, comminuted fractures, fragments, osteotomies, arthrodesis and bone grafts of the upper extremity, fibula, knee, ankle and foot in the presence of appropriate brace and/or immobilization.
OSSIO’s Compression Screw Portfolio will initially comprise a 4.0mm-diameter cannulated, headless, partially threaded compression screw in lengths ranging from 26mm to 60mm. Commercial launch will begin in early 2021.
OSSIOfiber uses bio-integrative material to provide stability and secure bone fixation while leaving no permanent hardware behind.
The company recently reached its 1,000th procedural milestone utilizing OSSIOfiber implants, including both OSSIOfiber Hammertoe Fixation (shown above) and the OSSIOfiber Trimmable Fixation Nail. Since 2019, U.S. surgeons have used the implants in a range of orthopedic procedures – including forefoot, midfoot, hindfoot and hand/wrist – with high satisfaction rates and no reported revision cases to date.
The bio-integrative implant is reportedly the only compression screw on the market that combines the necessary strength for bone fixation with the ability to fully integrate into the surrounding anatomy without adverse foreign body reactions and stress shielding, and avoids potential patient discomfort or implant related complications that are often associated with permanent metal hardware.
Brian Verrier, CEO, said, “The achievement of our 1,000th implant milestone, along with the recent FDA clearance for our Compression Screw Portfolio, further showcase our commitment to changing a 100-year-old standard-of-care in orthopedic fixation, transforming the patient experience and improving the overall healthcare economics of orthopedics.”
The company intends to pursue multiple applications for OSSIOfiber in the distal extremity, trauma, sports, reconstruction, pediatrics and spine segments.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







