
 Copy to clipboard
Copy to clipboard 
New navigation systems are entering the market to provide real-time, image-guided visualization that increases surgical accuracy, reduces radiation exposure and supports minimally invasive and complex procedures. These technologies aim to combine next-generation imaging with sleeker systems for spine surgery.
“Spinal navigation has been around since the 1990s, yet adoption has stalled around 30%,” said Paul Slosar, M.D., a renowned spine surgeon who serves as a strategic advisor to VUZE Medical.
He noted numerous reasons for the limited uptake in navigation, including minimal use in short-segment surgeries, overall capital costs, long learning curves and set-up times in the O.R. Additionally, Dr. Slosar said most mainstream navigation systems rely on optical tracking and have not significantly advanced over the last 30 years.
New navigation and imaging systems seek to simplify use of the technology by tapping into equipment already in the O.R., offering ASC-friendly sizes, and in some cases, including artificial intelligence (AI) applications. Below, we highlight three startups in the space.
See All AI
See All AI’s platform transforms standard 2D C-arm fluoroscopic X-rays into navigable 3D models with 1mm cross-sectional slices, eliminating the need for pre-operative CTs, MRIs, other scans or additional intraoperative imaging systems.
The platform is designed to work with existing fluoroscopy units that are paired with the company’s camera and AI computing software to create a 3D imaging and navigation solution. The system requires no direct line of sight between the company’s proprietary visible light camera and the patient’s anatomy, providing surgeons with 3D visualization of the patient’s internal anatomy without the need to create an open exposure.
The See All system is designated for minimally invasive surgery workflows. The company sees a significant advantage by tapping into the 150,000 fluoroscopy units already deployed around the world. Earlier this year, See All AI announced that it raised $33 million to fund development and commercialization efforts. The company plans to submit a 510(k) to FDA in 2025.
The company’s leadership team has significant experience in the space. Founder Gene Gregerson previously developed and sold the O-arm to Medtronic and the Airo system to Stryker. Company CEO Eric Major founded and sold K2M to Stryker. CMO Todd Albert serves as Surgeon-in-Chief Emeritus at the Hospital for Special Surgery.
HJY Medical
HJY’s flagship product is the VisualNext Endoscopic System, which incorporates high-quality imaging and AI computing to offer precision and efficiency in neuro and orthopedic diagnostic and surgical procedures.
The 2D/3D system incorporates semiconductor sensors, LEDs and an ISP system to eliminate the need for costly optics and enable single-use applications. The company says the system eliminates issues associated with reusable endoscopes, and its high-quality visualization shortens the learning curve for endoscopic surgery. It’s considered the market’s first AI-assisted endoscopic system.
VisualNext’s 2D system received U.S. FDA 510(k) clearance in 2023 and China NMPA clearance in 2024. R&D and regulatory efforts are underway on the AI-assisted device, which is expected to be commercialized in 2026.
HJY is headquartered in Taiwan and operates subsidiaries in China and the U.S. The company also offers a head-mounted display for surgical use. Founder and CEO Dr. John Chen is considered a pioneer in the development and commercialization of technologies for minimally invasive and endoscopic spine surgery in China and Taiwan.
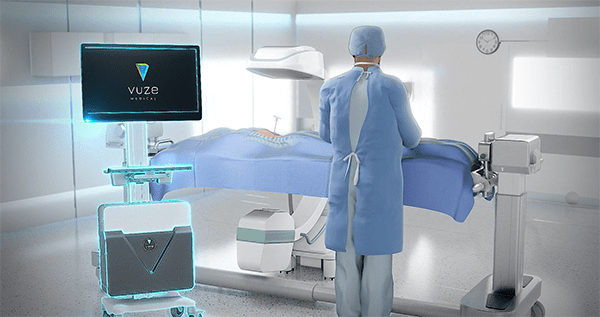
VUZE Medical
VUZE is a software solution installed on an off-the-shelf PC. It operates with unmodified surgical tools, uses no markers, references or cameras, and makes 3D imaging in the O.R. entirely optional. Using proprietary image processing algorithms, it overlays in real time graphical representations of standard surgical tools seen in intra-operative 2D X-ray images onto axial and sagittal cross-sections that it generates from the patient’s standard pre-operative 3D scan.
The second-generation VUZE System supports a broad range of surgical C-arms from all major vendors and accommodates numerous sources of 3D image data, including standard pre-operative CT and in-O.R. CT/CBCT scans. Its expanded functionality includes the ability to perform surgical planning at any time or place on a compatible standalone laptop in addition to the VUZE System itself.
VUZE Medical received FDA 510(k) clearance to market its second-generation VUZE System in 2024. While the system’s focus is minimally invasive thoracolumbar stabilizations, the underlying technology is not specific to particular anatomy, and the company intends to seek regulatory clearances for further spinal and skeletal interventions in the future.
VUZE has received related patents in the U.S., Europe, China and India, including tie-ins with robotics, augmented reality and traditional hardware-based navigation.
The company has announced $12.2 million in funding and is actively raising between $2 million and $5 million. The Israel-based VUZE Medical is led by CEO David Tolkowsky, who previously sold a non-orthopedic navigation system to Medtronic and an imaging system that is now part of Philips.
New navigation systems are entering the market to provide real-time, image-guided visualization that increases surgical accuracy, reduces radiation exposure and supports minimally invasive and complex procedures. These technologies aim to combine next-generation imaging with sleeker systems for spine surgery.
“Spinal navigation has been...
New navigation systems are entering the market to provide real-time, image-guided visualization that increases surgical accuracy, reduces radiation exposure and supports minimally invasive and complex procedures. These technologies aim to combine next-generation imaging with sleeker systems for spine surgery.
“Spinal navigation has been around since the 1990s, yet adoption has stalled around 30%,” said Paul Slosar, M.D., a renowned spine surgeon who serves as a strategic advisor to VUZE Medical.
He noted numerous reasons for the limited uptake in navigation, including minimal use in short-segment surgeries, overall capital costs, long learning curves and set-up times in the O.R. Additionally, Dr. Slosar said most mainstream navigation systems rely on optical tracking and have not significantly advanced over the last 30 years.
New navigation and imaging systems seek to simplify use of the technology by tapping into equipment already in the O.R., offering ASC-friendly sizes, and in some cases, including artificial intelligence (AI) applications. Below, we highlight three startups in the space.
See All AI
See All AI’s platform transforms standard 2D C-arm fluoroscopic X-rays into navigable 3D models with 1mm cross-sectional slices, eliminating the need for pre-operative CTs, MRIs, other scans or additional intraoperative imaging systems.
The platform is designed to work with existing fluoroscopy units that are paired with the company’s camera and AI computing software to create a 3D imaging and navigation solution. The system requires no direct line of sight between the company’s proprietary visible light camera and the patient’s anatomy, providing surgeons with 3D visualization of the patient’s internal anatomy without the need to create an open exposure.
The See All system is designated for minimally invasive surgery workflows. The company sees a significant advantage by tapping into the 150,000 fluoroscopy units already deployed around the world. Earlier this year, See All AI announced that it raised $33 million to fund development and commercialization efforts. The company plans to submit a 510(k) to FDA in 2025.
The company’s leadership team has significant experience in the space. Founder Gene Gregerson previously developed and sold the O-arm to Medtronic and the Airo system to Stryker. Company CEO Eric Major founded and sold K2M to Stryker. CMO Todd Albert serves as Surgeon-in-Chief Emeritus at the Hospital for Special Surgery.
HJY Medical
HJY’s flagship product is the VisualNext Endoscopic System, which incorporates high-quality imaging and AI computing to offer precision and efficiency in neuro and orthopedic diagnostic and surgical procedures.
The 2D/3D system incorporates semiconductor sensors, LEDs and an ISP system to eliminate the need for costly optics and enable single-use applications. The company says the system eliminates issues associated with reusable endoscopes, and its high-quality visualization shortens the learning curve for endoscopic surgery. It’s considered the market’s first AI-assisted endoscopic system.
VisualNext’s 2D system received U.S. FDA 510(k) clearance in 2023 and China NMPA clearance in 2024. R&D and regulatory efforts are underway on the AI-assisted device, which is expected to be commercialized in 2026.
HJY is headquartered in Taiwan and operates subsidiaries in China and the U.S. The company also offers a head-mounted display for surgical use. Founder and CEO Dr. John Chen is considered a pioneer in the development and commercialization of technologies for minimally invasive and endoscopic spine surgery in China and Taiwan.
VUZE Medical
VUZE is a software solution installed on an off-the-shelf PC. It operates with unmodified surgical tools, uses no markers, references or cameras, and makes 3D imaging in the O.R. entirely optional. Using proprietary image processing algorithms, it overlays in real time graphical representations of standard surgical tools seen in intra-operative 2D X-ray images onto axial and sagittal cross-sections that it generates from the patient’s standard pre-operative 3D scan.
The second-generation VUZE System supports a broad range of surgical C-arms from all major vendors and accommodates numerous sources of 3D image data, including standard pre-operative CT and in-O.R. CT/CBCT scans. Its expanded functionality includes the ability to perform surgical planning at any time or place on a compatible standalone laptop in addition to the VUZE System itself.
VUZE Medical received FDA 510(k) clearance to market its second-generation VUZE System in 2024. While the system’s focus is minimally invasive thoracolumbar stabilizations, the underlying technology is not specific to particular anatomy, and the company intends to seek regulatory clearances for further spinal and skeletal interventions in the future.
VUZE has received related patents in the U.S., Europe, China and India, including tie-ins with robotics, augmented reality and traditional hardware-based navigation.
The company has announced $12.2 million in funding and is actively raising between $2 million and $5 million. The Israel-based VUZE Medical is led by CEO David Tolkowsky, who previously sold a non-orthopedic navigation system to Medtronic and an imaging system that is now part of Philips.

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
CL
Carolyn LaWell is ORTHOWORLD's Chief Content Officer. She joined ORTHOWORLD in 2012 to oversee its editorial and industry education. She previously served in editor roles at B2B magazines and newspapers.