
 Copy to clipboard
Copy to clipboard 
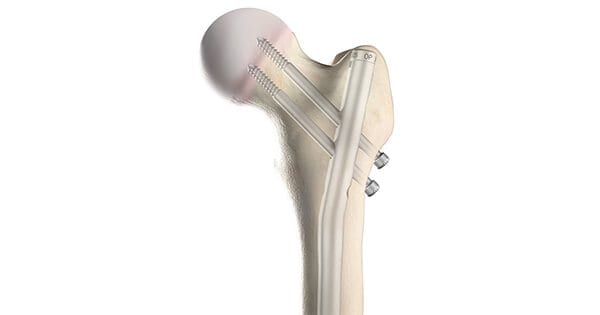
OrthoPediatrics gained FDA 510(k) clearance and commenced limited U.S. launch of its first sterile-packed implants for the Pediatric Nailing Platform | FEMUR (PNP|FEMUR).
The system, now available in 4 countries, was designed for use in pediatric patients to address femoral shaft fractures, subtrochanteric femur fractures, ipsilateral neck/shaft fractures, prophylactic nailing of impending pathologic fractures, nonunions, malunions, and fixation of femurs that have been surgically prepared for correction of deformity.
Worldwide, PNP|FEMUR has been used to treat over 1,000 children since its launch in 2018.
Joe Hauser, OrthoPediatrics’ Vice President of Trauma & Deformity Correction, said, “The introduction of sterile implants represents an important addition to our portfolio of sterile implant offerings. We are committed to adding sterile implants to the systems that can provide real value to our customers. We are strategically aligned with our customers to decrease the overall footprint of all our systems while balancing and ensuring intraoperative efficiency.”
OrthoPediatrics gained FDA 510(k) clearance and commenced limited U.S. launch of its first sterile-packed implants for the Pediatric Nailing Platform | FEMUR (PNP|FEMUR).
The system, now available in 4 countries, was designed for use in pediatric patients to address femoral shaft fractures, subtrochanteric femur fractures, ipsilateral...
OrthoPediatrics gained FDA 510(k) clearance and commenced limited U.S. launch of its first sterile-packed implants for the Pediatric Nailing Platform | FEMUR (PNP|FEMUR).
The system, now available in 4 countries, was designed for use in pediatric patients to address femoral shaft fractures, subtrochanteric femur fractures, ipsilateral neck/shaft fractures, prophylactic nailing of impending pathologic fractures, nonunions, malunions, and fixation of femurs that have been surgically prepared for correction of deformity.
Worldwide, PNP|FEMUR has been used to treat over 1,000 children since its launch in 2018.
Joe Hauser, OrthoPediatrics’ Vice President of Trauma & Deformity Correction, said, “The introduction of sterile implants represents an important addition to our portfolio of sterile implant offerings. We are committed to adding sterile implants to the systems that can provide real value to our customers. We are strategically aligned with our customers to decrease the overall footprint of all our systems while balancing and ensuring intraoperative efficiency.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







