Copy to clipboard
Copy to clipboard 
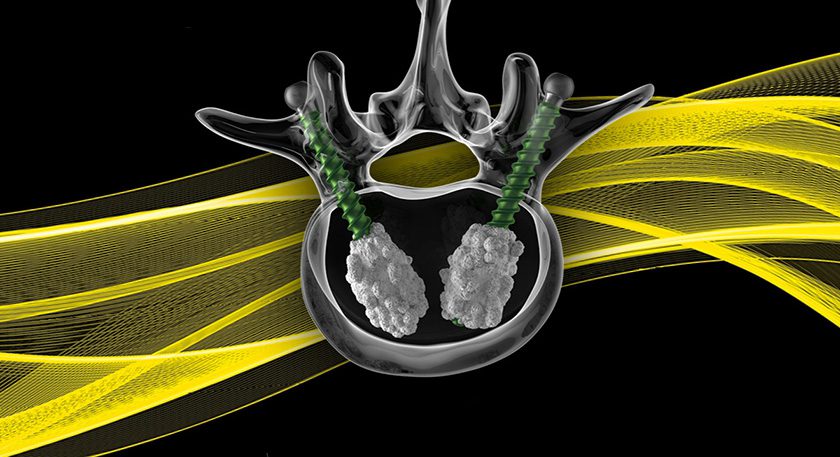
Life Spine received FDA 510(k) clearance to market the PROLIFT® Expandable Interbody System for use in minimally invasive PLIF, TLIF and oblique approaches.
PROLIFT is scheduled for limited release in 2Q16, with full product release expected later in 2016.
Source: Life Spine
Other interbody systems news thus far in 2016 includes:
Life Spine received FDA 510(k) clearance to market the PROLIFT® Expandable Interbody System for use in minimally invasive PLIF, TLIF and oblique approaches.
PROLIFT is scheduled for limited release in 2Q16, with full product release expected later in 2016.
Source: Life Spine
Other interbody systems news thus far in 2016...
Life Spine received FDA 510(k) clearance to market the PROLIFT® Expandable Interbody System for use in minimally invasive PLIF, TLIF and oblique approaches.
PROLIFT is scheduled for limited release in 2Q16, with full product release expected later in 2016.
Source: Life Spine
Other interbody systems news thus far in 2016 includes:
- Medtronic Launches AVILA Interbody Fusion Devices in the EU
- Spine Wave Licenses Patents from Atlas Spine
- Wenzel Spine Receives FDA 510(k) Clearance for VariLift-LX
- Amendia Launches OmegaLIF™ Expandable Interbody Cage
- Amedica Launches Valeo II Cervical Interbody System

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.