Copy to clipboard
Copy to clipboard 
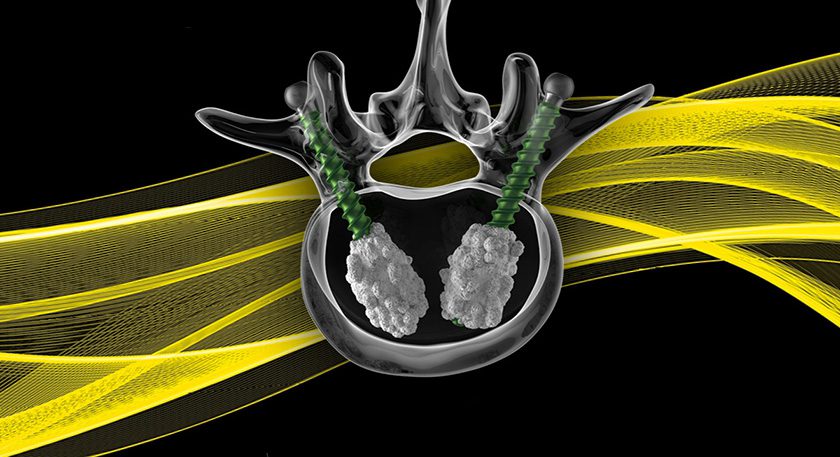
Wenzel Spine received FDA 510(k) clearance to market VariLift®-LX as a lumbar interbody fusion (IBF) device for standalone use.
Limited U.S. launch will commence in coming weeks, in advance of full launch planned for early spring 2016.
VariLift-LX is implanted in a unilateral or bilateral capacity via a PLIF or TLIF approach, and may be used standalone (without supplemental fixation). Wenzel now offers eleven sizes of VariLift devices.
Source: Wenzel Spine, Inc.
Wenzel Spine received its first 510(k) in 2010, and the cervical version of VariLift launched in the U.S. and EU in early 2013. In 2015, study results demonstrated long-term effectiveness of standalone IBF using VariLift, with an ability to obtain excellent clinical outcomes across multiple sites.
Wenzel Spine received FDA 510(k) clearance to market VariLift®-LX as a lumbar interbody fusion (IBF) device for standalone use.
Limited U.S. launch will commence in coming weeks, in advance of full launch planned for early spring 2016.
VariLift-LX is implanted in a unilateral or bilateral capacity via a PLIF or TLIF approach, and may be...
Wenzel Spine received FDA 510(k) clearance to market VariLift®-LX as a lumbar interbody fusion (IBF) device for standalone use.
Limited U.S. launch will commence in coming weeks, in advance of full launch planned for early spring 2016.
VariLift-LX is implanted in a unilateral or bilateral capacity via a PLIF or TLIF approach, and may be used standalone (without supplemental fixation). Wenzel now offers eleven sizes of VariLift devices.
Source: Wenzel Spine, Inc.
Wenzel Spine received its first 510(k) in 2010, and the cervical version of VariLift launched in the U.S. and EU in early 2013. In 2015, study results demonstrated long-term effectiveness of standalone IBF using VariLift, with an ability to obtain excellent clinical outcomes across multiple sites.

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.