Copy to clipboard
Copy to clipboard 
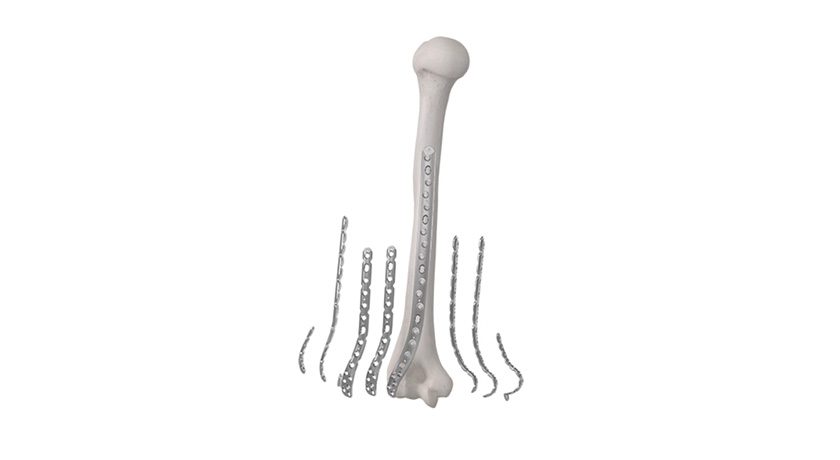
Intelligent Implant Systems received FDA 510(k) clearance to market 2-level components for the Revolution™ Spinal System, allowing for use in 2-level posterior thoracolumbar fusion.
The all-sterile implants are accompanied by a single tray of all-sterile instruments, eliminating the need for the hospital or ASC to sterilize anything for the procedure. The 2-level system utilizes existing Revolution™ Connectors, and requires only a minimum of additional components vs. the 1-level system.
Source: Intelligent Implant Systems, LLC
Intelligent Implant Systems received FDA 510(k) clearance to market 2-level components for the Revolution™ Spinal System, allowing for use in 2-level posterior thoracolumbar fusion.
The all-sterile implants are accompanied by a single tray of all-sterile instruments, eliminating the need for the hospital or ASC to...
Intelligent Implant Systems received FDA 510(k) clearance to market 2-level components for the Revolution™ Spinal System, allowing for use in 2-level posterior thoracolumbar fusion.
The all-sterile implants are accompanied by a single tray of all-sterile instruments, eliminating the need for the hospital or ASC to sterilize anything for the procedure. The 2-level system utilizes existing Revolution™ Connectors, and requires only a minimum of additional components vs. the 1-level system.
Source: Intelligent Implant Systems, LLC

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.