
 Copy to clipboard
Copy to clipboard 
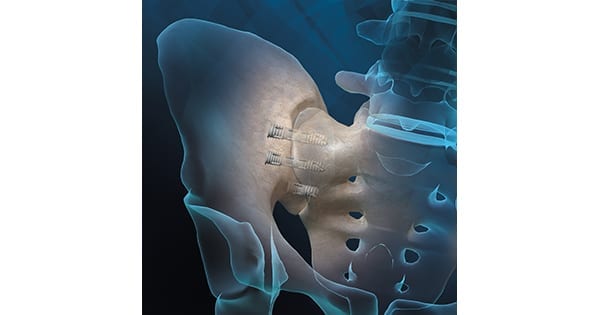
Orthofix Medical received FDA 510(k) clearance for the NANOVATE™ nanotechnology feature of the FIREBIRD™ SI Fusion system. Initially cleared and launched earlier this year, FIREBIRD SI Fusion is reportedly the first 3D-printed titanium bone screw with nanotechnology specifically designed to compress and stabilize the sacroiliac joint (SI joint) during fusion.
The device is packed with autograft and/or allograft, like MTF Biologics’ Trinity ELITE, to promote fusion. FIREBIRD SI screws are available in an assortment of lengths and diameters.
Orthofix Medical received FDA 510(k) clearance for the NANOVATE™ nanotechnology feature of the FIREBIRD™ SI Fusion system. Initially cleared and launched earlier this year, FIREBIRD SI Fusion is reportedly the first 3D-printed titanium bone screw with nanotechnology specifically designed to compress and stabilize the sacroiliac joint (SI...
Orthofix Medical received FDA 510(k) clearance for the NANOVATE™ nanotechnology feature of the FIREBIRD™ SI Fusion system. Initially cleared and launched earlier this year, FIREBIRD SI Fusion is reportedly the first 3D-printed titanium bone screw with nanotechnology specifically designed to compress and stabilize the sacroiliac joint (SI joint) during fusion.
The device is packed with autograft and/or allograft, like MTF Biologics’ Trinity ELITE, to promote fusion. FIREBIRD SI screws are available in an assortment of lengths and diameters.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







