
 Copy to clipboard
Copy to clipboard 
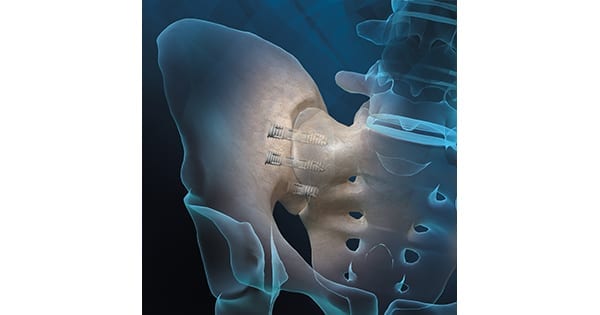
Orthofix Medical received FDA 510(k) clearance to market the FIREBIRD™ SI Fusion System. First procedures have taken place during limited U.S. launch.
Designed to compress and stabilize the sacroiliac (SI) joint, FIREBIRD SI Fusion is the first 3D-printed titanium bone screw to launch in the U.S. for the treatment of SI joint dysfunction.
FIREBIRD SI features a porous mid-shaft region that allows bone to grow into its surface. With a cannulated screw design, the system allows the surgeon to pack the device with autograft and/or allografts, like the Trinity ELITE™ Allograft with viable cells, to help ensure bone fusion. The FIREBIRD SI screws are available in an assortment of lengths and diameters.
“Orthofix Spine prides itself in creating differentiated technology for the advancement of patient care,” said Kevin Kenny, Global President of Orthofix Spine. “We are excited to bring the FIREBIRD SI Fusion System to market as an extension of our flagship FIREBIRD line of devices. This new system is another example of our commitment to providing surgeons and their patients with innovative options that interface with products and solutions within our portfolio.”
Orthofix Medical received FDA 510(k) clearance to market the FIREBIRD™ SI Fusion System. First procedures have taken place during limited U.S. launch.
Designed to compress and stabilize the sacroiliac (SI) joint, FIREBIRD SI Fusion is the first 3D-printed titanium bone screw to launch in the U.S. for the treatment of SI joint dysfunction.
...
Orthofix Medical received FDA 510(k) clearance to market the FIREBIRD™ SI Fusion System. First procedures have taken place during limited U.S. launch.
Designed to compress and stabilize the sacroiliac (SI) joint, FIREBIRD SI Fusion is the first 3D-printed titanium bone screw to launch in the U.S. for the treatment of SI joint dysfunction.
FIREBIRD SI features a porous mid-shaft region that allows bone to grow into its surface. With a cannulated screw design, the system allows the surgeon to pack the device with autograft and/or allografts, like the Trinity ELITE™ Allograft with viable cells, to help ensure bone fusion. The FIREBIRD SI screws are available in an assortment of lengths and diameters.
“Orthofix Spine prides itself in creating differentiated technology for the advancement of patient care,” said Kevin Kenny, Global President of Orthofix Spine. “We are excited to bring the FIREBIRD SI Fusion System to market as an extension of our flagship FIREBIRD line of devices. This new system is another example of our commitment to providing surgeons and their patients with innovative options that interface with products and solutions within our portfolio.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







