
 Copy to clipboard
Copy to clipboard 
NuVasive received its latest FDA 510(k) clearance for the Pulse® platform, after receiving approval under the CE Mark earlier this year. Further, the company announced the commercial launch of Pulse which is now available for sale in targeted global regions.
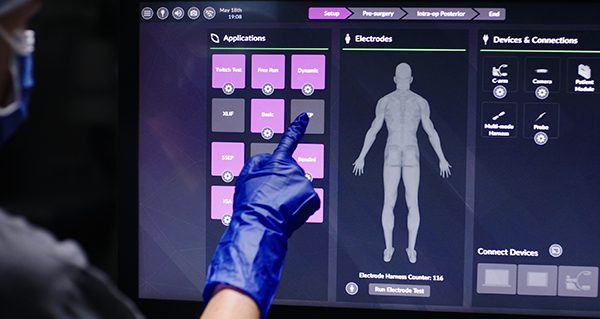
Pulse is an integrated technology platform designed to increase safety, efficiency, and procedural reproducibility of spine surgery. The platform allows surgeons to easily access multiple technologies from a condensed footprint and address some of the most common surgical challenges. It is currently the only enabling technology platform with the ability for utilization in 100% of spine procedures.
The benefits of less invasive surgery are supported by extensive clinical evidence, and include:
- Reduced OR time by up to 60 minutes
- Savings of nearly $5,000 per patient in hospital costs
- Reduced time under anesthesia and lower intraoperative risks
- Reduced length of stay in the hospital
Pulse integrates multiple technologies into one platform, and its architecture can support future applications, including robotics and smart tools. Currently, the platform includes:
Radiation reduction and imaging enhancement: NuVasive’s Lessray® technology was designed to increase OR efficiency through streamlined imaging workflow, while also significantly reducing exposure to radiation for everyone in the room. Pulse supports numerous imaging systems, but offers enhanced integration with Siemens’ cutting-edge 3D mobile C-arm—the Cios Spin®.
Navigation: Pulse introduces a procedurally integrated navigation technology that offers the potential to improve screw placement accuracy and minimize radiation.
Neuromonitoring: This application provides proprietary automated nerve detection with standardized setup and clinically validated alerts to help reduce variability and allow for faster interpretation of neural information.
Global alignment: NuVasive’s Integrated Global Alignment (iGA®) technology offers surgical planning and intraoperative assessment tools to help surgeons correct or restore spinal alignment. Surgeons can later follow up postoperatively to assess the results of the procedure.
Patient-specific rod bending: NuVasive’s spinal rod bending technology, Bendini®, is used to create patient-specific rods that are bent to implant locations. Bendini expedites manual rod manipulation through computer-assisted bend instructions.
Wireless connectivity: This tool allows seamless connectivity and control of the Pulse platform from all members of the surgical team in the OR—from the surgeon to the C-arm technologist to support staff.
Source: NuVasive, Inc.
NuVasive received its latest FDA 510(k) clearance for the Pulse® platform, after receiving approval under the CE Mark earlier this year. Further, the company announced the commercial launch of Pulse which is now available for sale in targeted global regions.
Pulse is an integrated technology platform designed to increase safety,...
NuVasive received its latest FDA 510(k) clearance for the Pulse® platform, after receiving approval under the CE Mark earlier this year. Further, the company announced the commercial launch of Pulse which is now available for sale in targeted global regions.
Pulse is an integrated technology platform designed to increase safety, efficiency, and procedural reproducibility of spine surgery. The platform allows surgeons to easily access multiple technologies from a condensed footprint and address some of the most common surgical challenges. It is currently the only enabling technology platform with the ability for utilization in 100% of spine procedures.
The benefits of less invasive surgery are supported by extensive clinical evidence, and include:
- Reduced OR time by up to 60 minutes
- Savings of nearly $5,000 per patient in hospital costs
- Reduced time under anesthesia and lower intraoperative risks
- Reduced length of stay in the hospital
Pulse integrates multiple technologies into one platform, and its architecture can support future applications, including robotics and smart tools. Currently, the platform includes:
Radiation reduction and imaging enhancement: NuVasive’s Lessray® technology was designed to increase OR efficiency through streamlined imaging workflow, while also significantly reducing exposure to radiation for everyone in the room. Pulse supports numerous imaging systems, but offers enhanced integration with Siemens’ cutting-edge 3D mobile C-arm—the Cios Spin®.
Navigation: Pulse introduces a procedurally integrated navigation technology that offers the potential to improve screw placement accuracy and minimize radiation.
Neuromonitoring: This application provides proprietary automated nerve detection with standardized setup and clinically validated alerts to help reduce variability and allow for faster interpretation of neural information.
Global alignment: NuVasive’s Integrated Global Alignment (iGA®) technology offers surgical planning and intraoperative assessment tools to help surgeons correct or restore spinal alignment. Surgeons can later follow up postoperatively to assess the results of the procedure.
Patient-specific rod bending: NuVasive’s spinal rod bending technology, Bendini®, is used to create patient-specific rods that are bent to implant locations. Bendini expedites manual rod manipulation through computer-assisted bend instructions.
Wireless connectivity: This tool allows seamless connectivity and control of the Pulse platform from all members of the surgical team in the OR—from the surgeon to the C-arm technologist to support staff.
Source: NuVasive, Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







