
 Copy to clipboard
Copy to clipboard 
NuVasive announced that the Pulse® platform received CE Mark approval for its latest design update and clinical evaluations are underway in multiple countries throughout Europe.
“The latest CE Mark approval and clinical evaluations are key commercialization milestones for the Pulse platform and further our plan for an expanded global launch later this summer,” said Massimo Calafiore, executive vice president, Global Business Units at NuVasive. “There is a substantial opportunity for enabling technology adoption in spine surgery, and we are leveraging our expertise in proceduralization to introduce Pulse. This next generation of enabling technology has applications that can be utilized in every spine procedure.”
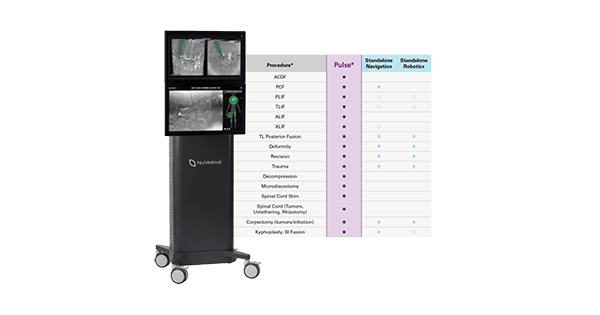
Pulse is a first-of-its-kind technology that integrates radiation reduction, imaging enhancement, rod bending, navigation, intraoperative neuromonitoring, and spinal alignment tools into a single platform. It is currently the only enabling technology platform with the ability for utilization in 100% of spine procedures and throughout the entire operating room (OR) workflow, allowing surgeons to easily access multiple technologies from a condensed footprint. Pulse is designed to increase safety, efficiency, and procedural reproducibility, while addressing some of the most common clinical challenges in spine surgery.
Source: NuVasive, Inc.
NuVasive announced that the Pulse® platform received CE Mark approval for its latest design update and clinical evaluations are underway in multiple countries throughout Europe.
"The latest CE Mark approval and clinical evaluations are key commercialization milestones for the Pulse platform and further our plan for an expanded global launch...
NuVasive announced that the Pulse® platform received CE Mark approval for its latest design update and clinical evaluations are underway in multiple countries throughout Europe.
“The latest CE Mark approval and clinical evaluations are key commercialization milestones for the Pulse platform and further our plan for an expanded global launch later this summer,” said Massimo Calafiore, executive vice president, Global Business Units at NuVasive. “There is a substantial opportunity for enabling technology adoption in spine surgery, and we are leveraging our expertise in proceduralization to introduce Pulse. This next generation of enabling technology has applications that can be utilized in every spine procedure.”
Pulse is a first-of-its-kind technology that integrates radiation reduction, imaging enhancement, rod bending, navigation, intraoperative neuromonitoring, and spinal alignment tools into a single platform. It is currently the only enabling technology platform with the ability for utilization in 100% of spine procedures and throughout the entire operating room (OR) workflow, allowing surgeons to easily access multiple technologies from a condensed footprint. Pulse is designed to increase safety, efficiency, and procedural reproducibility, while addressing some of the most common clinical challenges in spine surgery.
Source: NuVasive, Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







