
 Copy to clipboard
Copy to clipboard 
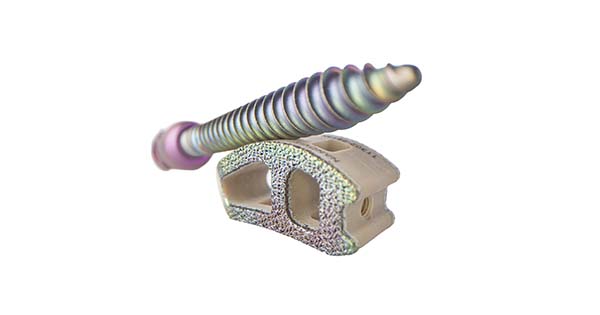
Nanovis announced the successful completion of its 500th surgery with FortiFix pedicle screws.
FortiFix are the first FDA-cleared pedicle screw with a nanotechnology designation. Over 3,000 pedicle screws have been implanted in the initial 500 surgeries, with patients experiencing positive outcomes, including no known infections or non-fusions noted in the Maude database. The system has been employed across a spectrum of surgeries, including degenerative, deformity, trauma and revision cases.
Nano FortiFix pedicle screws were launched in late 2021.
Source: Nanovis
Nanovis announced the successful completion of its 500th surgery with FortiFix pedicle screws.
FortiFix are the first FDA-cleared pedicle screw with a nanotechnology designation. Over 3,000 pedicle screws have been implanted in the initial 500 surgeries, with patients experiencing positive outcomes, including no known infections or non-fusions...
Nanovis announced the successful completion of its 500th surgery with FortiFix pedicle screws.
FortiFix are the first FDA-cleared pedicle screw with a nanotechnology designation. Over 3,000 pedicle screws have been implanted in the initial 500 surgeries, with patients experiencing positive outcomes, including no known infections or non-fusions noted in the Maude database. The system has been employed across a spectrum of surgeries, including degenerative, deformity, trauma and revision cases.
Nano FortiFix pedicle screws were launched in late 2021.
Source: Nanovis

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







