
 Copy to clipboard
Copy to clipboard 
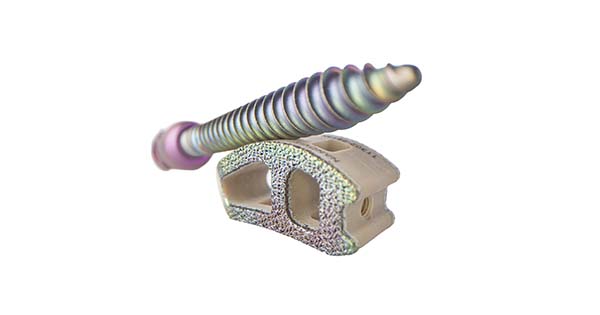
Nanovis announced the first implantations of its Bioceramic Nanotube-Enhanced pedicle screw system, Nano FortiFix, the only nanotechnology designated titanium-surfaced pedicle screw.
Nano FortiFix pedicle screws are designed with a proprietary and patent protected bio-ceramic nanotube surface. Unlike other technologies, Nanovis’s bioceramic nanotubes were granted a nanotechnology designation with specific label language stating “The nanotube arrays have been shown to increase and accelerate calcified extracellular matrix production in vitro.” The Nano FortiFix label also includes statistically significantly superior human osteoblast and mesenchymal stem cell calcified extracellular matrix production data compared to other surfaces.
Nanovis was the first company to receive nanotechnology designation for PEEK enhanced titanium interbodies and is the first and only company to receive nanotechnology designation for titanium-surfaced pedicle screws.
This is Nanovis’ 5th implant system cleared with a bioceramic nanotube surface establishing a well understood regulatory pathway. Nanovis’ commitment to help reduce the pain and suffering from implant loosening has never been higher and we are grateful to the surgeons, scientists, and employees that have created such a powerful combination of technology-enhanced systems.
Source: Nanovis
Nanovis announced the first implantations of its Bioceramic Nanotube-Enhanced pedicle screw system, Nano FortiFix, the only nanotechnology designated titanium-surfaced pedicle screw.
Nano FortiFix pedicle screws are designed with a proprietary and patent protected bio-ceramic nanotube surface. Unlike other technologies, Nanovis's bioceramic...
Nanovis announced the first implantations of its Bioceramic Nanotube-Enhanced pedicle screw system, Nano FortiFix, the only nanotechnology designated titanium-surfaced pedicle screw.
Nano FortiFix pedicle screws are designed with a proprietary and patent protected bio-ceramic nanotube surface. Unlike other technologies, Nanovis’s bioceramic nanotubes were granted a nanotechnology designation with specific label language stating “The nanotube arrays have been shown to increase and accelerate calcified extracellular matrix production in vitro.” The Nano FortiFix label also includes statistically significantly superior human osteoblast and mesenchymal stem cell calcified extracellular matrix production data compared to other surfaces.
Nanovis was the first company to receive nanotechnology designation for PEEK enhanced titanium interbodies and is the first and only company to receive nanotechnology designation for titanium-surfaced pedicle screws.
This is Nanovis’ 5th implant system cleared with a bioceramic nanotube surface establishing a well understood regulatory pathway. Nanovis’ commitment to help reduce the pain and suffering from implant loosening has never been higher and we are grateful to the surgeons, scientists, and employees that have created such a powerful combination of technology-enhanced systems.
Source: Nanovis

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







