Copy to clipboard
Copy to clipboard 
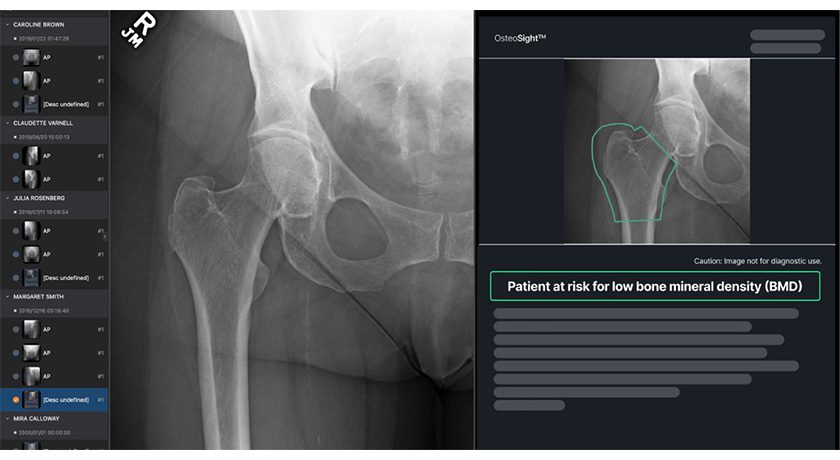
NanoHive Medical secured the exclusive sublicense of DirectSync Surgical’s piezoelectric implantable sensor technology for the field of spinal fusion. DirectSync Surgical had licensed the technology from the University of Kansas.
NanoHive is exploring the advancement of successful proof-of-concept research to potentially develop bone stimulating and remote monitoring/data collection sensor technology housed in the company’s spinal interbody fusion device lattice which is currently commercialized in the company’s Hive™ Soft Titanium® portfolio of 3D printed spinal interbody fusion devices.
In October 2023, the company reported the initiation of a research collaboration with DirectSync Surgical. Based on the preliminary research generated, the company ascertained that their proprietary rhombic dodecahedron lattice technology was uniquely capable of transferring significant energy from the encapsulated piezoelectric sensor. The company is currently in the process of working with FDA on the transfer of the Breakthrough Device Designation from DirectSync to NanoHive.
Patrick O’Donnell, President & CEO of NanoHive, stated, “NanoHive’s Soft Titanium® technology is the ideal interbody fusion implant to deliver a sensor with data collection and bone stimulation capability. The company is excited about the prospect of leading the way with the development of a technological leap in the interbody fusion device category. Our view of the future of innovation in spine devices emphasizes that new technologies must demonstrate an improvement to patient virtual care and clinical outcomes as well as connectivity with enabling surgical technologies and artificial intelligence. These advancements must also deliver cost-efficiencies to the various stakeholders involved in the spine patient’s episode-of-care. More meaningful and frequent data collection that can ultimately be utilized as a predictive tool will be essential to this vision.”
Source: NanoHive Medical LLC
NanoHive Medical secured the exclusive sublicense of DirectSync Surgical’s piezoelectric implantable sensor technology for the field of spinal fusion. DirectSync Surgical had licensed the technology from the University of Kansas.
NanoHive is exploring the advancement of successful proof-of-concept research to potentially develop bone...
NanoHive Medical secured the exclusive sublicense of DirectSync Surgical’s piezoelectric implantable sensor technology for the field of spinal fusion. DirectSync Surgical had licensed the technology from the University of Kansas.
NanoHive is exploring the advancement of successful proof-of-concept research to potentially develop bone stimulating and remote monitoring/data collection sensor technology housed in the company’s spinal interbody fusion device lattice which is currently commercialized in the company’s Hive™ Soft Titanium® portfolio of 3D printed spinal interbody fusion devices.
In October 2023, the company reported the initiation of a research collaboration with DirectSync Surgical. Based on the preliminary research generated, the company ascertained that their proprietary rhombic dodecahedron lattice technology was uniquely capable of transferring significant energy from the encapsulated piezoelectric sensor. The company is currently in the process of working with FDA on the transfer of the Breakthrough Device Designation from DirectSync to NanoHive.
Patrick O’Donnell, President & CEO of NanoHive, stated, “NanoHive’s Soft Titanium® technology is the ideal interbody fusion implant to deliver a sensor with data collection and bone stimulation capability. The company is excited about the prospect of leading the way with the development of a technological leap in the interbody fusion device category. Our view of the future of innovation in spine devices emphasizes that new technologies must demonstrate an improvement to patient virtual care and clinical outcomes as well as connectivity with enabling surgical technologies and artificial intelligence. These advancements must also deliver cost-efficiencies to the various stakeholders involved in the spine patient’s episode-of-care. More meaningful and frequent data collection that can ultimately be utilized as a predictive tool will be essential to this vision.”
Source: NanoHive Medical LLC

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.