
 Copy to clipboard
Copy to clipboard 
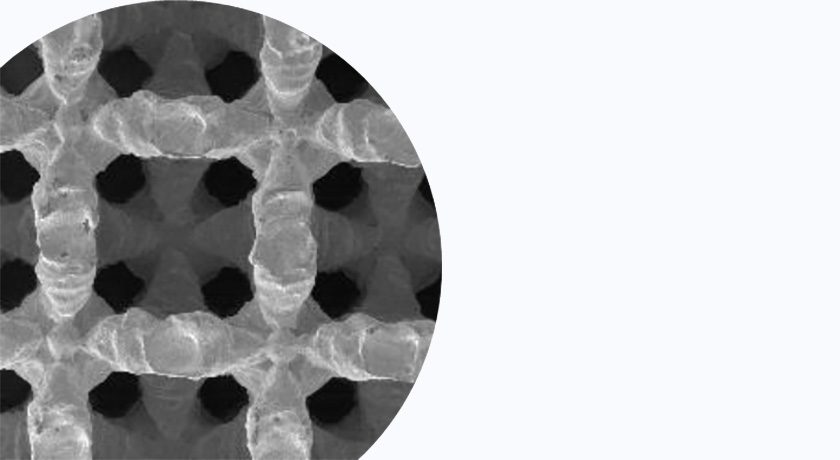
NanoHive Medical and DirectSync Surgical are partnering to develop a first-of-kind stimulating/sensing 3D-printed interbody fusion cage.
The central objectives of the research comprise two key initiatives. The first strives to seamlessly integrate patient-powered stimulation into Hive™ implants, with the goal of expediting fusion and potentially mitigating costs associated with biologics and patient compliance issues associated with external bone growth stimulators.
The second objective is to equip surgeons with a critical post-operative data analysis tool. Moreover, this innovation has the potential to reduce expenses associated with post-operative imaging.
Patrick O’Donnell, CEO of NanoHive Medical, said, “NanoHive’s Soft Titanium® technology is the ideal interbody fusion implant to be combined with DirectSync’s bone stimulation and diagnostic sensor technology. We are excited about collaborating on the development of what we believe is a potential technology leap in the interbody fusion device category.”
Zygmunt Porada, CEO of DirectSync Surgical, adds, “DirectSync Surgical is proud to collaborate with NanoHive Medical on this exciting endeavor. Our combined expertise will empower surgeons with devices that provide to them objective data insights to enable improved patient outcomes and reduce costs to the healthcare system.”
Source: NanoHive Medical LLC
NanoHive Medical and DirectSync Surgical are partnering to develop a first-of-kind stimulating/sensing 3D-printed interbody fusion cage.
The central objectives of the research comprise two key initiatives. The first strives to seamlessly integrate patient-powered stimulation into Hive™ implants, with the goal of expediting fusion and...
NanoHive Medical and DirectSync Surgical are partnering to develop a first-of-kind stimulating/sensing 3D-printed interbody fusion cage.
The central objectives of the research comprise two key initiatives. The first strives to seamlessly integrate patient-powered stimulation into Hive™ implants, with the goal of expediting fusion and potentially mitigating costs associated with biologics and patient compliance issues associated with external bone growth stimulators.
The second objective is to equip surgeons with a critical post-operative data analysis tool. Moreover, this innovation has the potential to reduce expenses associated with post-operative imaging.
Patrick O’Donnell, CEO of NanoHive Medical, said, “NanoHive’s Soft Titanium® technology is the ideal interbody fusion implant to be combined with DirectSync’s bone stimulation and diagnostic sensor technology. We are excited about collaborating on the development of what we believe is a potential technology leap in the interbody fusion device category.”
Zygmunt Porada, CEO of DirectSync Surgical, adds, “DirectSync Surgical is proud to collaborate with NanoHive Medical on this exciting endeavor. Our combined expertise will empower surgeons with devices that provide to them objective data insights to enable improved patient outcomes and reduce costs to the healthcare system.”
Source: NanoHive Medical LLC

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







