
 Copy to clipboard
Copy to clipboard 
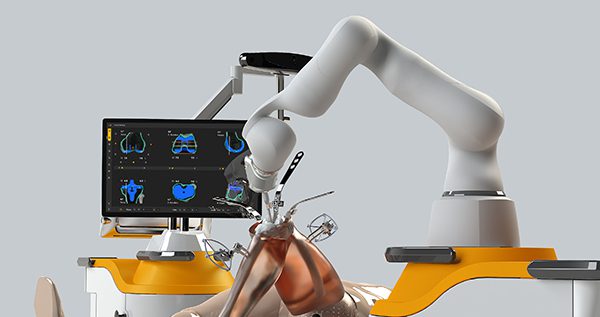
Monogram Technologies announced completion of the first-ever fully autonomous saw-based robotic knee replacement procedure on a live patient. The procedure was performed at the Krishna Shalby Hospital in Ahmedabad, India, as part of Monogram’s multi-center clinical trial in India for the Monogram TKA System.
As previously announced, Monogram and Shalby partnered to evaluate the safety and effectiveness of the mBôs TKA System with the Consensus CKS implant, which is substantially equivalent to the Monogram mPress implants for regulatory purposes. On April 29, 2025, Monogram obtained regulatory approval from India’s Central Drugs Standard Control Organization to import its mBôs TKA system to conduct a multi-center clinical investigation evaluating the safety and effectiveness of the Monogram TKA System. The clinical trial includes 102 total knee replacement procedures, with a three-month clinical follow-up, conducted across multiple sites in India.
FDA recently granted 510(k) clearance for Monogram’s mBôs semi-autonomous TKA System.
Zimmer Biomet and Monogram Technologies recently announced that they entered into a definitive agreement for Zimmer Biomet to acquire all outstanding shares of stock of Monogram.
“The first fully autonomous knee replacement on a live patient using Monogram’s TKA System is a tremendous milestone for the Company. The procedure marks the culmination of years of hard work and dedication by our incredible team. I feel honored and proud to work with such incredible and dedicated individuals at Monogram. The system appears to have flawlessly executed the plan based on intraoperative surgeon evaluation, including intraoperative laxity estimation and achievement of planned alignment. I believe Monogram’s product will have a significant positive impact on the standard of care for orthopedic medicine in the years to come,” said Benjamin Sexson, CEO of Monogram Technologies.
“Watching the ease with which the system cut and executed the procedure was incredibly fulfilling. The safety and accuracy of the system were on obvious display. It really was an amazing moment watching Dr. Patil realize that robots can be more than marketing gimmicks but tools that actually change how knee replacements can be done better, said founder Dr. Douglas Unis. “Based on years of evaluating technology, watching this surgery was the final validation I needed to know with certainty that we have a groundbreaking product. It was incredible that there was so little stress, given the level of autonomy and first-time use on a living person. It was flawless.”
Source: Monogram Technologies Inc.
Monogram Technologies announced completion of the first-ever fully autonomous saw-based robotic knee replacement procedure on a live patient. The procedure was performed at the Krishna Shalby Hospital in Ahmedabad, India, as part of Monogram’s multi-center clinical trial in India for the Monogram TKA System.
As previously announced,...
Monogram Technologies announced completion of the first-ever fully autonomous saw-based robotic knee replacement procedure on a live patient. The procedure was performed at the Krishna Shalby Hospital in Ahmedabad, India, as part of Monogram’s multi-center clinical trial in India for the Monogram TKA System.
As previously announced, Monogram and Shalby partnered to evaluate the safety and effectiveness of the mBôs TKA System with the Consensus CKS implant, which is substantially equivalent to the Monogram mPress implants for regulatory purposes. On April 29, 2025, Monogram obtained regulatory approval from India’s Central Drugs Standard Control Organization to import its mBôs TKA system to conduct a multi-center clinical investigation evaluating the safety and effectiveness of the Monogram TKA System. The clinical trial includes 102 total knee replacement procedures, with a three-month clinical follow-up, conducted across multiple sites in India.
FDA recently granted 510(k) clearance for Monogram’s mBôs semi-autonomous TKA System.
Zimmer Biomet and Monogram Technologies recently announced that they entered into a definitive agreement for Zimmer Biomet to acquire all outstanding shares of stock of Monogram.
“The first fully autonomous knee replacement on a live patient using Monogram’s TKA System is a tremendous milestone for the Company. The procedure marks the culmination of years of hard work and dedication by our incredible team. I feel honored and proud to work with such incredible and dedicated individuals at Monogram. The system appears to have flawlessly executed the plan based on intraoperative surgeon evaluation, including intraoperative laxity estimation and achievement of planned alignment. I believe Monogram’s product will have a significant positive impact on the standard of care for orthopedic medicine in the years to come,” said Benjamin Sexson, CEO of Monogram Technologies.
“Watching the ease with which the system cut and executed the procedure was incredibly fulfilling. The safety and accuracy of the system were on obvious display. It really was an amazing moment watching Dr. Patil realize that robots can be more than marketing gimmicks but tools that actually change how knee replacements can be done better, said founder Dr. Douglas Unis. “Based on years of evaluating technology, watching this surgery was the final validation I needed to know with certainty that we have a groundbreaking product. It was incredible that there was so little stress, given the level of autonomy and first-time use on a living person. It was flawless.”
Source: Monogram Technologies Inc.

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







