
 Copy to clipboard
Copy to clipboard 
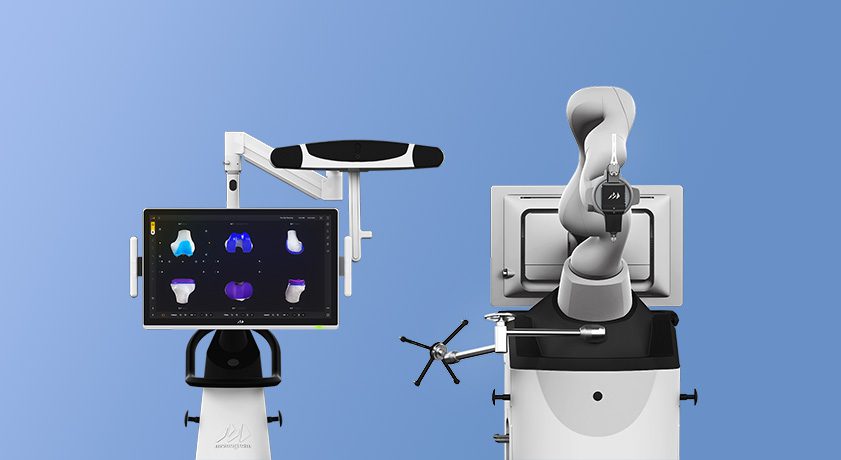
Monogram Technologies entered into a strategic collaboration with Shalby Limited, a global muti-specialty hospital chain and one of India’s leading orthopedic hospital groups, to conduct a multicenter clinical trial to demonstrate the safety and effectiveness of the mBȏs TKA System.
Shalby is reportedly ranked #1 in arthroplasty by volume in the world. It employs over 4,000 people at 14 hospitals in 13 cities, with more than three million patients treated. Shalby represents approximately 15% of the organized arthroplasty market in India, which has an approximate market size of 200,000 TKAs per year (with a compound annual growth rate of approximately 20% and a population of 1.4 billion people). Shalby, which has been growing at a 20% fifteen-year CAGR, currently operates in 8 countries with aggressive multi-continent international expansion plans. In 2021, Shalby purchased Consensus Orthopedics and established a U.S. presence.
Under the collaboration, Shalby will enroll patients at various sites in India for surgeons to evaluate the safety and effectiveness of the mBȏs TKA System with the Consensus CKS implant, which is substantially equivalent to the Monogram mPress implants for regulatory purposes. Monogram received comments on the Clinical Investigational Plan during its February 2024 Presubmission Communications with FDA and has incorporated feedback into its 510(k) application submitted to FDA on July 19, 2024 and which passed the FDA Administrative Review. Notably, the strategic collaboration contemplates the post-trial transfer of a robot to the hospital system under certain conditions following the trial as the companies contemplate further collaboration.
Monogram plans to leverage the clinical data from the U.S. study for post-launch marketing and to support international clearance and commercialization. Both companies see a significant clinical need for a highly intuitive, safe and accurate multi-application robotic platform. The companies expect the relationship to extend, with additional studies on next-generation solutions like mVision to follow.
Source: Shalby Limited and Monogram Technologies Inc.
Monogram Technologies entered into a strategic collaboration with Shalby Limited, a global muti-specialty hospital chain and one of India's leading orthopedic hospital groups, to conduct a multicenter clinical trial to demonstrate the safety and effectiveness of the mBȏs TKA System.
Shalby is reportedly ranked #1 in arthroplasty by volume...
Monogram Technologies entered into a strategic collaboration with Shalby Limited, a global muti-specialty hospital chain and one of India’s leading orthopedic hospital groups, to conduct a multicenter clinical trial to demonstrate the safety and effectiveness of the mBȏs TKA System.
Shalby is reportedly ranked #1 in arthroplasty by volume in the world. It employs over 4,000 people at 14 hospitals in 13 cities, with more than three million patients treated. Shalby represents approximately 15% of the organized arthroplasty market in India, which has an approximate market size of 200,000 TKAs per year (with a compound annual growth rate of approximately 20% and a population of 1.4 billion people). Shalby, which has been growing at a 20% fifteen-year CAGR, currently operates in 8 countries with aggressive multi-continent international expansion plans. In 2021, Shalby purchased Consensus Orthopedics and established a U.S. presence.
Under the collaboration, Shalby will enroll patients at various sites in India for surgeons to evaluate the safety and effectiveness of the mBȏs TKA System with the Consensus CKS implant, which is substantially equivalent to the Monogram mPress implants for regulatory purposes. Monogram received comments on the Clinical Investigational Plan during its February 2024 Presubmission Communications with FDA and has incorporated feedback into its 510(k) application submitted to FDA on July 19, 2024 and which passed the FDA Administrative Review. Notably, the strategic collaboration contemplates the post-trial transfer of a robot to the hospital system under certain conditions following the trial as the companies contemplate further collaboration.
Monogram plans to leverage the clinical data from the U.S. study for post-launch marketing and to support international clearance and commercialization. Both companies see a significant clinical need for a highly intuitive, safe and accurate multi-application robotic platform. The companies expect the relationship to extend, with additional studies on next-generation solutions like mVision to follow.
Source: Shalby Limited and Monogram Technologies Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







