
 Copy to clipboard
Copy to clipboard 
Medartis went public on the SIX Swiss Exchange in March, securing gross proceeds, including overallotment of shares, of CHF 142.6MM (translated to US $150.48MM as of the transaction date of 3/22/18) to fund product development, international expansion and potential acquisitions. Their public launch provided deeper insight into the company’s position within the global orthopaedic trauma space. We reviewed Medartis’ 2017 and 2018 strategic announcements, and spoke with leadership to get a better sense of the company’s recent and future movement.
We asked Willi Miesch, who has held the title of CEO since the beginning of the company’s 21 years in existence, why go public?
“Medartis has a proven track record of successful market penetration based on next-generation osteosynthesis products in upper and lower extremities and CMF. We are targeting the fastest growing segments in an $8 billion market,” Mr. Miesch said. “We were ready for the next step to accelerate geographic and product expansion. With the IPO, we wanted to sharpen our profile in the market and to increase our financial flexibility to take advantage of the growth opportunities we see ahead of us.”
 |
PRODUCT PORTFOLIO EXPANSION
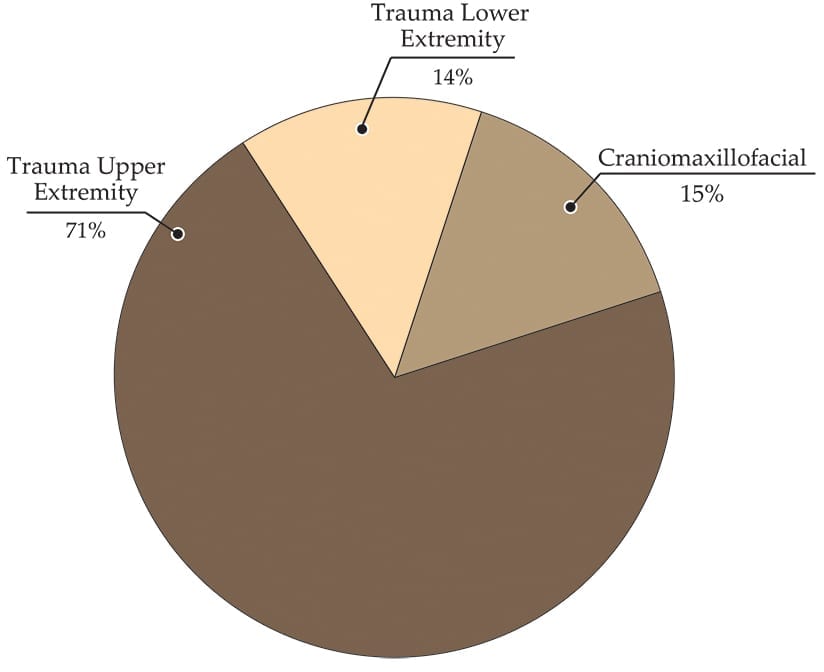
We estimate that Medartis is the 10th largest player in the global orthopaedic trauma market, with 1% share. Its trauma line comprises APTUS implants for hand, wrist, elbow, shoulder and foot & ankle indications.
Medartis reported 2017 net revenue of ~$107.2MM, +14% vs. 2016. For 1H18, Medartis reported revenue of $62.2MM +23% vs. 1H17, Trauma $52.9MM, +25%: Upper Extremity $44.4MM, +19%; Lower Extremity $8.5MM, +43%; Other (craniomaxillofacial) $9.4MM, + 29%
In 1H18, Medartis launched additional wrist plates to address specific fracture patterns. The company also announced its first shoulder product. The APTUS Proximal Humerus System 3.5 emphasizes screw optimization gained from experience in lower extremities implant development with higher load bearing requirements. Full launch is expected in 1Q19.
Medartis’ lower extremity priorities include increasing its network and education programs for foot and ankle surgeons, as well as developing products to fill portfolio gaps.
 |
GEOGRAPHIC EXPANSION
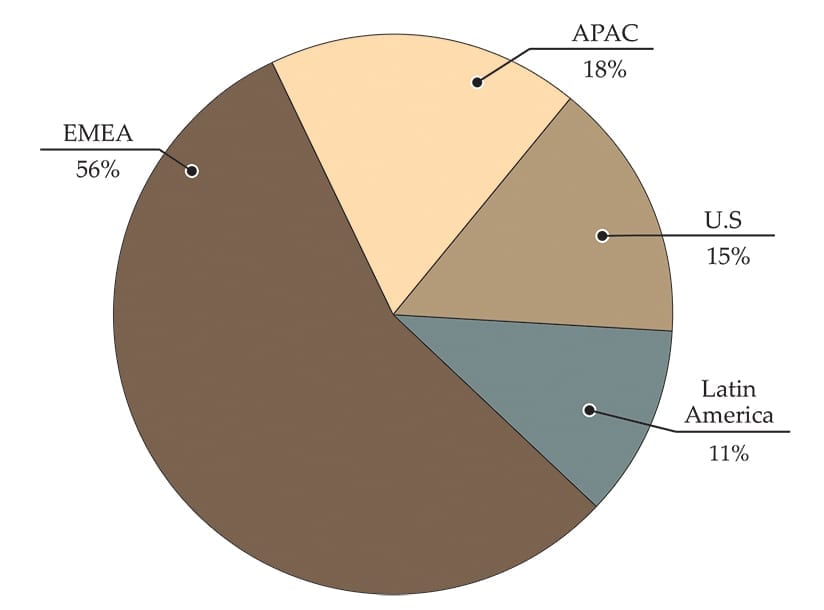
Medartis sells products in 52 countries, with more than half of its revenue coming from the EMEA region.
The company’s 1H18 geographic revenue vs 1H17 included:
- U.S. $9.5MM, +10%
- Ex-U.S. $52.7MM, +29%
- EMEA $34.7MM, +21%
- APAC $11.4MM, +17%
- Rest of World (Latin America) $6.6MM, +85%
We asked Mr. Miesch about the market forces driving Medartis’ business in Europe, as well as the company’s planned expansion in Brazil, China and Japan.
Regarding Europe, Mr. Miesch said, “Generally speaking, the specialization in surgery to solve more complex clinical problems is progressing rapidly, which also increases the demand for specialized solutions such as those offered by Medartis. This is especially true for the lower extremities market which is at an earlier stage of the growth cycle compared to the upper extremities. Specifically with regard to Europe, the implementation of new regulatory requirements [ISO 13485:2016 and MDR] requires high professional expertise and the necessary resources. We have a well-established business in Europe, and are in a very good position to further strengthen our market position in this region.”
In 2017, Medartis purchased its Brazil distributor, Extera. The integration is almost complete and the company plans to expand its salesforce in the country, according to Mr. Miesch.
Medartis’ 2018 priorities include opening a subsidiary in Japan and preparing for market entry in China. The Japanese subsidiary is expected to start operations by year-end, selling lower extremity products. Medartis will continue to sell its upper extremities products through its long-term Japanese distributor. Regarding China, Mr. Miesch said that the company is on track with its plans but declined to provide specific timing for market entry.
POTENTIAL ACQUISITIONS
Medartis disclosed two acquisitions in 2017—Extera and the purchase of Swiss software startup Mimedis.
The latter will allow Medartis to offer preoperative planning and patient specific implants. Prior to the transaction, Mimedis supported hospitals in Switzerland and Germany with preoperative planning and manufacturing of 3D models, especially in craniomaxillofacial procedures.
While Medartis emphasized the acquisition’s ability to enhance its craniomaxillofacial portfolio, we think that the technology could also be leveraged in the future for trauma cases.
The IPO expanded Medartis’ financial capabilities, which will support pursuit of additional acquisition opportunities. Mr. Miesch indicated that Medartis is evaluating those that complement its product portfolio and geographic presence.
We’ve outlined the current Medartis profile. What does Medartis look like five years from now? We expect that the expansion of its shoulder and foot device lines, two high-growth areas in orthopaedics, will provide Medartis with a more complete portfolio as it seeks to penetrate current markets and emerging high-growth markets.
Mr. Miesch expects the company to climb on our list of global trauma players.
“Our goal is to become a global top three player in major small bone markets. We already achieved this in some relevant markets as in Germany or Australia, and we are well on track to further strengthen our position in other markets,” he said. “We started with Medartis 20 years ago, and continue to be very excited to reach out into untapped geographies and to develop innovative solutions. I am sure we will have the same spirit for many years to come.”
MEDARTIS BY THE NUMBERS
- Founded: 1997
- Entered Upper Extremities: 2004
- Entered Lower Extremities: 2010
- 2017 net revenue: $107.2MM
- Employees: 500+
- Locations: 12
- Patent families pending or granted: 20
- Market Ranking: Claims #1 or #2 Position in Germany, Austria, Switzerland, France and Australia
Carolyn LaWell is ORTHOWORLD’s Chief Content Officer. She can be reached by email.
Medartis went public on the SIX Swiss Exchange in March, securing gross proceeds, including overallotment of shares, of CHF 142.6MM (translated to US $150.48MM as of the transaction date of 3/22/18) to fund product development, international expansion and potential acquisitions. Their public launch provided deeper insight into the company’s...
Medartis went public on the SIX Swiss Exchange in March, securing gross proceeds, including overallotment of shares, of CHF 142.6MM (translated to US $150.48MM as of the transaction date of 3/22/18) to fund product development, international expansion and potential acquisitions. Their public launch provided deeper insight into the company’s position within the global orthopaedic trauma space. We reviewed Medartis’ 2017 and 2018 strategic announcements, and spoke with leadership to get a better sense of the company’s recent and future movement.
We asked Willi Miesch, who has held the title of CEO since the beginning of the company’s 21 years in existence, why go public?
“Medartis has a proven track record of successful market penetration based on next-generation osteosynthesis products in upper and lower extremities and CMF. We are targeting the fastest growing segments in an $8 billion market,” Mr. Miesch said. “We were ready for the next step to accelerate geographic and product expansion. With the IPO, we wanted to sharpen our profile in the market and to increase our financial flexibility to take advantage of the growth opportunities we see ahead of us.”
 |
PRODUCT PORTFOLIO EXPANSION
We estimate that Medartis is the 10th largest player in the global orthopaedic trauma market, with 1% share. Its trauma line comprises APTUS implants for hand, wrist, elbow, shoulder and foot & ankle indications.
Medartis reported 2017 net revenue of ~$107.2MM, +14% vs. 2016. For 1H18, Medartis reported revenue of $62.2MM +23% vs. 1H17, Trauma $52.9MM, +25%: Upper Extremity $44.4MM, +19%; Lower Extremity $8.5MM, +43%; Other (craniomaxillofacial) $9.4MM, + 29%
In 1H18, Medartis launched additional wrist plates to address specific fracture patterns. The company also announced its first shoulder product. The APTUS Proximal Humerus System 3.5 emphasizes screw optimization gained from experience in lower extremities implant development with higher load bearing requirements. Full launch is expected in 1Q19.
Medartis’ lower extremity priorities include increasing its network and education programs for foot and ankle surgeons, as well as developing products to fill portfolio gaps.
 |
GEOGRAPHIC EXPANSION
Medartis sells products in 52 countries, with more than half of its revenue coming from the EMEA region.
The company’s 1H18 geographic revenue vs 1H17 included:
- U.S. $9.5MM, +10%
- Ex-U.S. $52.7MM, +29%
- EMEA $34.7MM, +21%
- APAC $11.4MM, +17%
- Rest of World (Latin America) $6.6MM, +85%
We asked Mr. Miesch about the market forces driving Medartis’ business in Europe, as well as the company’s planned expansion in Brazil, China and Japan.
Regarding Europe, Mr. Miesch said, “Generally speaking, the specialization in surgery to solve more complex clinical problems is progressing rapidly, which also increases the demand for specialized solutions such as those offered by Medartis. This is especially true for the lower extremities market which is at an earlier stage of the growth cycle compared to the upper extremities. Specifically with regard to Europe, the implementation of new regulatory requirements [ISO 13485:2016 and MDR] requires high professional expertise and the necessary resources. We have a well-established business in Europe, and are in a very good position to further strengthen our market position in this region.”
In 2017, Medartis purchased its Brazil distributor, Extera. The integration is almost complete and the company plans to expand its salesforce in the country, according to Mr. Miesch.
Medartis’ 2018 priorities include opening a subsidiary in Japan and preparing for market entry in China. The Japanese subsidiary is expected to start operations by year-end, selling lower extremity products. Medartis will continue to sell its upper extremities products through its long-term Japanese distributor. Regarding China, Mr. Miesch said that the company is on track with its plans but declined to provide specific timing for market entry.
POTENTIAL ACQUISITIONS
Medartis disclosed two acquisitions in 2017—Extera and the purchase of Swiss software startup Mimedis.
The latter will allow Medartis to offer preoperative planning and patient specific implants. Prior to the transaction, Mimedis supported hospitals in Switzerland and Germany with preoperative planning and manufacturing of 3D models, especially in craniomaxillofacial procedures.
While Medartis emphasized the acquisition’s ability to enhance its craniomaxillofacial portfolio, we think that the technology could also be leveraged in the future for trauma cases.
The IPO expanded Medartis’ financial capabilities, which will support pursuit of additional acquisition opportunities. Mr. Miesch indicated that Medartis is evaluating those that complement its product portfolio and geographic presence.
We’ve outlined the current Medartis profile. What does Medartis look like five years from now? We expect that the expansion of its shoulder and foot device lines, two high-growth areas in orthopaedics, will provide Medartis with a more complete portfolio as it seeks to penetrate current markets and emerging high-growth markets.
Mr. Miesch expects the company to climb on our list of global trauma players.
“Our goal is to become a global top three player in major small bone markets. We already achieved this in some relevant markets as in Germany or Australia, and we are well on track to further strengthen our position in other markets,” he said. “We started with Medartis 20 years ago, and continue to be very excited to reach out into untapped geographies and to develop innovative solutions. I am sure we will have the same spirit for many years to come.”
MEDARTIS BY THE NUMBERS
- Founded: 1997
- Entered Upper Extremities: 2004
- Entered Lower Extremities: 2010
- 2017 net revenue: $107.2MM
- Employees: 500+
- Locations: 12
- Patent families pending or granted: 20
- Market Ranking: Claims #1 or #2 Position in Germany, Austria, Switzerland, France and Australia
Carolyn LaWell is ORTHOWORLD’s Chief Content Officer. She can be reached by email.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
CL
Carolyn LaWell is ORTHOWORLD's Chief Content Officer. She joined ORTHOWORLD in 2012 to oversee its editorial and industry education. She previously served in editor roles at B2B magazines and newspapers.







