
 Copy to clipboard
Copy to clipboard 
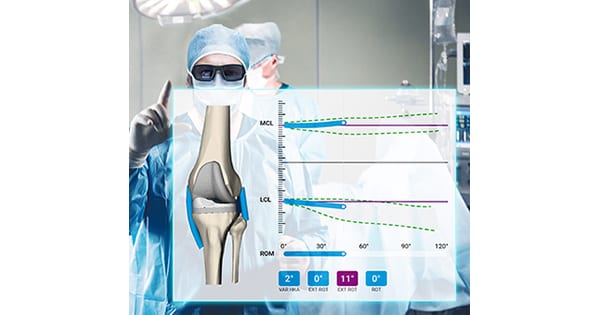
Medacta received approval under the CE Mark for NextAR™ knee, shoulder and spine applications as part of its NextAR Augmented Reality (AR) surgical platform. Also, the NextAR shoulder application received FDA 510(k) clearance, expanding their U.S. offering from the knee application which received clearance in 2020.
NextAR’s smart delivery tools include the single-use NextAR TS tracking system and NextAR Augmented Reality Smart Glasses, and is the first platform to offer Augmented Reality solutions for both spine and joint replacement. NextAR is part of the MySolutions ecosystem of implants and surgical techniques,
CEO Francesco Siccardi said, “With our NextAR Platform we wanted to take another step forward in personalized medicine, improving accuracy in computer-assisted surgery. Efficiency in the operating room is crucial for surgeons and hospitals, and NextAR has the potential to provide significant benefits to healthcare systems around the world. We are proud to have developed an extremely versatile platform, with a single, compact hardware that applies to both joint and spine applications. NextAR perfectly fits in Medacta’s vision and in our sophisticated MySolutions personalized ecosystem, focused on delivering advanced personalized solutions that support the surgeon’s care of each patient.”
Medacta received approval under the CE Mark for NextAR™ knee, shoulder and spine applications as part of its NextAR Augmented Reality (AR) surgical platform. Also, the NextAR shoulder application received FDA 510(k) clearance, expanding their U.S. offering from the knee application which received clearance in 2020.
NextAR's smart...
Medacta received approval under the CE Mark for NextAR™ knee, shoulder and spine applications as part of its NextAR Augmented Reality (AR) surgical platform. Also, the NextAR shoulder application received FDA 510(k) clearance, expanding their U.S. offering from the knee application which received clearance in 2020.
NextAR’s smart delivery tools include the single-use NextAR TS tracking system and NextAR Augmented Reality Smart Glasses, and is the first platform to offer Augmented Reality solutions for both spine and joint replacement. NextAR is part of the MySolutions ecosystem of implants and surgical techniques,
CEO Francesco Siccardi said, “With our NextAR Platform we wanted to take another step forward in personalized medicine, improving accuracy in computer-assisted surgery. Efficiency in the operating room is crucial for surgeons and hospitals, and NextAR has the potential to provide significant benefits to healthcare systems around the world. We are proud to have developed an extremely versatile platform, with a single, compact hardware that applies to both joint and spine applications. NextAR perfectly fits in Medacta’s vision and in our sophisticated MySolutions personalized ecosystem, focused on delivering advanced personalized solutions that support the surgeon’s care of each patient.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







