
 Copy to clipboard
Copy to clipboard 
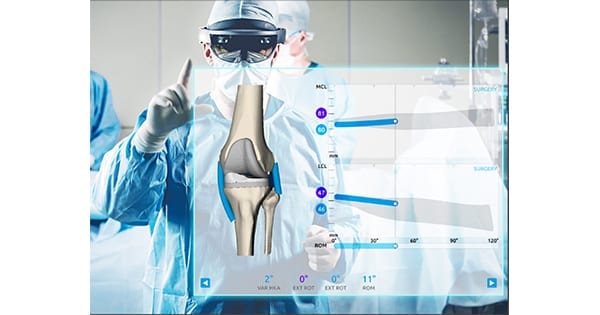
Medacta received FDA clearance to market its NextAR™ Augmented Reality based surgical platform for total knee arthroplasty (TKA).
NextAR TKA, which works with the Medacta GMK Sphere Medially Stabilized Knee, is the first application of a new platform technology which will be extended to hip, shoulder and spine, and is designed with Artificial Intelligence and Machine Learning to add efficiency and precision to pre-operative CT-based planning and analysis.
Single-use NextAR TKA requires a low upfront capital investment from clinics and hospitals, and is appropriate for use in ambulatory surgical centers. Augmented Reality glasses allow the surgeon to visualize surgical actions and information in real-time, directly on the operative field, to retain patient focus and track 3D soft tissue behavior during a surgery in real time.
In addition to planning tools, Medacta has developed NextAR TS, an infrared single-use tracking system that can enhance surgical efficiency and support execution of pre-op plans.
Francesco Siccardi, CEO of Medacta, said, “Patients and surgeons are attracted by improved outcomes and innovation, the NextAR Platform is one Medacta answer to the current race in orthopaedic technology and I am very excited about this milestone achievement. Requiring a very limited investment in capital equipment, the NextAR Platform perfectly represents Medacta’s commitment to develop solutions that are able to improve patient outcomes and healthcare system sustainability.”
Medacta received FDA clearance to market its NextAR™ Augmented Reality based surgical platform for total knee arthroplasty (TKA).
NextAR TKA, which works with the Medacta GMK Sphere Medially Stabilized Knee, is the first application of a new platform technology which will be extended to hip, shoulder and spine, and is designed with Artificial...
Medacta received FDA clearance to market its NextAR™ Augmented Reality based surgical platform for total knee arthroplasty (TKA).
NextAR TKA, which works with the Medacta GMK Sphere Medially Stabilized Knee, is the first application of a new platform technology which will be extended to hip, shoulder and spine, and is designed with Artificial Intelligence and Machine Learning to add efficiency and precision to pre-operative CT-based planning and analysis.
Single-use NextAR TKA requires a low upfront capital investment from clinics and hospitals, and is appropriate for use in ambulatory surgical centers. Augmented Reality glasses allow the surgeon to visualize surgical actions and information in real-time, directly on the operative field, to retain patient focus and track 3D soft tissue behavior during a surgery in real time.
In addition to planning tools, Medacta has developed NextAR TS, an infrared single-use tracking system that can enhance surgical efficiency and support execution of pre-op plans.
Francesco Siccardi, CEO of Medacta, said, “Patients and surgeons are attracted by improved outcomes and innovation, the NextAR Platform is one Medacta answer to the current race in orthopaedic technology and I am very excited about this milestone achievement. Requiring a very limited investment in capital equipment, the NextAR Platform perfectly represents Medacta’s commitment to develop solutions that are able to improve patient outcomes and healthcare system sustainability.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.