
 Copy to clipboard
Copy to clipboard 
The aging global population, representing a significant number of people who suffer from low back pain and poor outcomes in spinal fusions, has led Locate Bio to focus on the advancement of bone graft substitutes in patients who suffer from degenerative disc disease.
Data has long shown the success of recombinant human bone morphogenetic protein-2 (rhBMP-2) in growing new bone, but has also exposed the severe side effects the protein can cause in patients. Locate Bio, a UK-based regenerative medicine startup, believes it has solved the challenges associated with rhBMP-2 when healing fractures.
“Nothing grows bone as well as BMP does,” said John von Benecke, Locate Bio’s CEO. “By addressing the root cause of the signal-saturation-causing side effects from over-presenting at early time points, the technology has the potential to become transformational.”
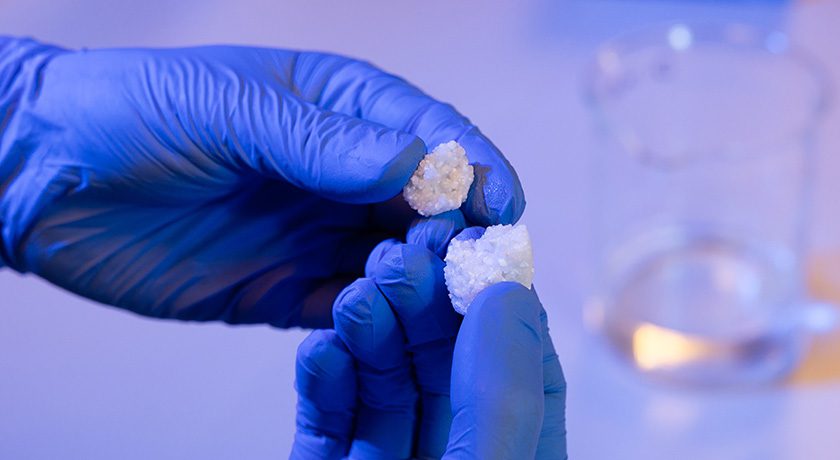
Locate Bio’s LDGraft is composed of cylindrical pellets, which comprise a biodegradable polymer that encapsulates the rhBMP-2. Once LDGraft is applied, the polymer degrades, releasing low doses of rhBMP-2 as the pellets provide a surface for cell attachment. The product eliminates the addition of liquid rhBMP-2 in the O.R., reliance on surface attachment to the protein and soak time-dependent rhBMP-2 dose variability.
LDGraft received FDA Breakthrough Device Designation in 2023 and was recently implanted in the first patient as part of the RESTORE study, a prospective randomized clinical trial evaluating the safety and efficacy of LDGraft in single-level anterior lumbar interbody fusion. The study’s launch is another milestone in the company’s journey to market.
Technology Advancements
Locate Bio was founded in 2001 as a spinout from The University of Nottingham’s School of Pharmacy to develop advanced drug delivery systems. The company’s history in biopharma and regenerative medicine led it to study alternatives for bone healing, including research in ceramics, stem cells and small molecules.
“Our view is that nothing beats a well-controlled BMP,” Mr. von Benecke said. “We spent many years looking at the other technologies before coming to that rationale.”
rhBMP-2 is naturally present in the body. Locate Bio scientists wanted to understand why its use in spinal fusion was linked to severe side effects like ectopic bone growth. Mr. von Benecke said companies often focus on just the retention of the protein. Locate Bio’s research found that was important, but perhaps more important was the oversaturation of the signal at the initial inflammatory phase of bone healing.
“We know that BMP follows a principal SMAD pathway for signaling. Along with bone regrowth, it can form fat cells (adipogenesis), overstimulate osteoclasts and cause inflammatory responses. There is a distinct delay in peak BMP expression in humans around one to two weeks,” Mr. von Benecke said. “We used that knowledge to temper the initial presentation of the molecule.
“It’s not just about retention; it’s making sure that we’re mimicking the natural expression of the molecule in fracture healing,” he added. “Because the BMP-2 is encased, the majority of the protein is shielded, and the body isn’t exposed to the total dose that’s implanted until we gradually release it over five weeks to avoid a massive saturation or drug burst.”
Locate Bio’s encapsulation of rhBMP-2 in a biodegradable material differentiates its technology from other products and tempers the availability of the molecule, especially during the high inflammatory phase of bone healing.
The company developed a proprietary manufacturing method using a poly(lactic-co-glycolic acid) base process to produce pellets. The BMP-2 encasing pellets contain hollow inner lumens and are co-packed with B-TCP granules to form a dry mixture. Saline is added in the O.R., which converts the product into a moldable putty ready for implantation without the need for soak time.
LDGraft is expected to save significant time in the O.R. compared to competitor products. Locate Bio plans to aggressively price the technology to take share from adjacent segments like stem cell, demineralized bone matrix and premium ceramic products.
Study Results
Mr. von Benecke said the company’s in vivo and in vitro studies show LDGraft’s advantages over products already on the market and in development.
In an in vitro model, the rhBMP-2 release was sustained for 28 days. In an in vivo study with 120 sheep, all three rhBMP-2 doses tested were equivalent to iliac crest bone graft in terms of fusion at six months and no side effects were identified.
Mr. von Benecke said the results were significant compared to competitors’ products. “Other companies have published data that showed there wasn’t good bone healing, or there were side effects,” he added. “We’re the only ones to have shown equivalence to iliac crest bone graft in a sheep interbody study without adverse effects.”
Locate Bio is currently enrolling patients in a clinical study in Australia that will evaluate both clinical fusion outcomes and patient-reported outcomes. The pilot study tests two dose levels versus the control. Mr. von Benecke said the results will support the dose range that Locate Bio uses in a forthcoming pivotal study.
The company completed an oversubscribed £9.2 million ($11.5 million) funding round earlier this year. The capital from new investors and existing investors, including Mercia Ventures and BGF, is allocated to support the clinical study.
“We have long believed in Locate Bio’s mission to develop next-generation orthobiologics that can transform patients’ lives,” Alex Gwyther, an investor at Mercia Ventures, said at the time of the funding announcement. “We are pleased to see both the progress in the technology and the world-class team that the company has brought together.”
Building for the Future
Locate Bio’s team and manufacturing facility is in Nottingham, England. Mr. von Benecke said its association with the University of Nottingham has allowed the company to attract young, bright students who have grown professionally and personally with the company. Nearly 80% of the company’s employees are women, and it has Investors in People (IIP) Platinum accreditation — cultural components of which the company is particularly proud.
About 95% of Locate Bio’s efforts are focused on moving LDGraft through clinical studies and the regulatory process, but the company also has two other products in its pipeline. CertOss is a ceramic bone graft and OsteoMime is being developed for revision spinal surgeries.
“We have a very strong group of people working hard,” Mr. von Benecke said, “to get this company where we need to go.”
The aging global population, representing a significant number of people who suffer from low back pain and poor outcomes in spinal fusions, has led Locate Bio to focus on the advancement of bone graft substitutes in patients who suffer from degenerative disc disease.
Data has long shown the success of recombinant human bone morphogenetic...
The aging global population, representing a significant number of people who suffer from low back pain and poor outcomes in spinal fusions, has led Locate Bio to focus on the advancement of bone graft substitutes in patients who suffer from degenerative disc disease.
Data has long shown the success of recombinant human bone morphogenetic protein-2 (rhBMP-2) in growing new bone, but has also exposed the severe side effects the protein can cause in patients. Locate Bio, a UK-based regenerative medicine startup, believes it has solved the challenges associated with rhBMP-2 when healing fractures.
“Nothing grows bone as well as BMP does,” said John von Benecke, Locate Bio’s CEO. “By addressing the root cause of the signal-saturation-causing side effects from over-presenting at early time points, the technology has the potential to become transformational.”
Locate Bio’s LDGraft is composed of cylindrical pellets, which comprise a biodegradable polymer that encapsulates the rhBMP-2. Once LDGraft is applied, the polymer degrades, releasing low doses of rhBMP-2 as the pellets provide a surface for cell attachment. The product eliminates the addition of liquid rhBMP-2 in the O.R., reliance on surface attachment to the protein and soak time-dependent rhBMP-2 dose variability.
LDGraft received FDA Breakthrough Device Designation in 2023 and was recently implanted in the first patient as part of the RESTORE study, a prospective randomized clinical trial evaluating the safety and efficacy of LDGraft in single-level anterior lumbar interbody fusion. The study’s launch is another milestone in the company’s journey to market.
Technology Advancements
Locate Bio was founded in 2001 as a spinout from The University of Nottingham’s School of Pharmacy to develop advanced drug delivery systems. The company’s history in biopharma and regenerative medicine led it to study alternatives for bone healing, including research in ceramics, stem cells and small molecules.
“Our view is that nothing beats a well-controlled BMP,” Mr. von Benecke said. “We spent many years looking at the other technologies before coming to that rationale.”
rhBMP-2 is naturally present in the body. Locate Bio scientists wanted to understand why its use in spinal fusion was linked to severe side effects like ectopic bone growth. Mr. von Benecke said companies often focus on just the retention of the protein. Locate Bio’s research found that was important, but perhaps more important was the oversaturation of the signal at the initial inflammatory phase of bone healing.
“We know that BMP follows a principal SMAD pathway for signaling. Along with bone regrowth, it can form fat cells (adipogenesis), overstimulate osteoclasts and cause inflammatory responses. There is a distinct delay in peak BMP expression in humans around one to two weeks,” Mr. von Benecke said. “We used that knowledge to temper the initial presentation of the molecule.
“It’s not just about retention; it’s making sure that we’re mimicking the natural expression of the molecule in fracture healing,” he added. “Because the BMP-2 is encased, the majority of the protein is shielded, and the body isn’t exposed to the total dose that’s implanted until we gradually release it over five weeks to avoid a massive saturation or drug burst.”
Locate Bio’s encapsulation of rhBMP-2 in a biodegradable material differentiates its technology from other products and tempers the availability of the molecule, especially during the high inflammatory phase of bone healing.
The company developed a proprietary manufacturing method using a poly(lactic-co-glycolic acid) base process to produce pellets. The BMP-2 encasing pellets contain hollow inner lumens and are co-packed with B-TCP granules to form a dry mixture. Saline is added in the O.R., which converts the product into a moldable putty ready for implantation without the need for soak time.
LDGraft is expected to save significant time in the O.R. compared to competitor products. Locate Bio plans to aggressively price the technology to take share from adjacent segments like stem cell, demineralized bone matrix and premium ceramic products.
Study Results
Mr. von Benecke said the company’s in vivo and in vitro studies show LDGraft’s advantages over products already on the market and in development.
In an in vitro model, the rhBMP-2 release was sustained for 28 days. In an in vivo study with 120 sheep, all three rhBMP-2 doses tested were equivalent to iliac crest bone graft in terms of fusion at six months and no side effects were identified.
Mr. von Benecke said the results were significant compared to competitors’ products. “Other companies have published data that showed there wasn’t good bone healing, or there were side effects,” he added. “We’re the only ones to have shown equivalence to iliac crest bone graft in a sheep interbody study without adverse effects.”
Locate Bio is currently enrolling patients in a clinical study in Australia that will evaluate both clinical fusion outcomes and patient-reported outcomes. The pilot study tests two dose levels versus the control. Mr. von Benecke said the results will support the dose range that Locate Bio uses in a forthcoming pivotal study.
The company completed an oversubscribed £9.2 million ($11.5 million) funding round earlier this year. The capital from new investors and existing investors, including Mercia Ventures and BGF, is allocated to support the clinical study.
“We have long believed in Locate Bio’s mission to develop next-generation orthobiologics that can transform patients’ lives,” Alex Gwyther, an investor at Mercia Ventures, said at the time of the funding announcement. “We are pleased to see both the progress in the technology and the world-class team that the company has brought together.”
Building for the Future
Locate Bio’s team and manufacturing facility is in Nottingham, England. Mr. von Benecke said its association with the University of Nottingham has allowed the company to attract young, bright students who have grown professionally and personally with the company. Nearly 80% of the company’s employees are women, and it has Investors in People (IIP) Platinum accreditation — cultural components of which the company is particularly proud.
About 95% of Locate Bio’s efforts are focused on moving LDGraft through clinical studies and the regulatory process, but the company also has two other products in its pipeline. CertOss is a ceramic bone graft and OsteoMime is being developed for revision spinal surgeries.
“We have a very strong group of people working hard,” Mr. von Benecke said, “to get this company where we need to go.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
CL
Carolyn LaWell is ORTHOWORLD's Chief Content Officer. She joined ORTHOWORLD in 2012 to oversee its editorial and industry education. She previously served in editor roles at B2B magazines and newspapers.







