
 Copy to clipboard
Copy to clipboard 
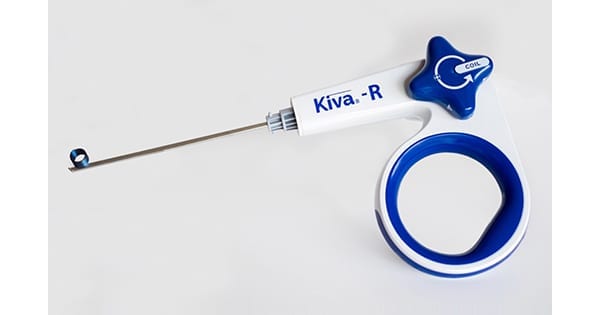
IZI Medical Products received approval under the CE Mark for the unipedicular PEEK implant-based Kiva™ Vertebral Compression Fracture (VCF) Treatment.
In two Level I clinical studies, Kiva was shown to reduce the rate of adjacent level fractures and cement extravasation and restore the kyphotic angle. The implant mimics cancellous bone and provides structural support to prevent further collapse of the vertebral body. The unipedicular deployment method allows midline placement to support the axial vertebral body load.
IZI Medical acquired Benvenue Medical’s vertebral augmentation systems portfolio in 2018, including the Kiva VCF system, Kiva Pilot and Blazer Vertebral Augmentation System.
“We are excited to provide this novel implant technology to the European market and have made significant investments in our international distribution network to broaden our VCF offering,” said Greg Groenke, Chief Executive Officer, IZI Medical.
IZI Medical Products received approval under the CE Mark for the unipedicular PEEK implant-based Kiva™ Vertebral Compression Fracture (VCF) Treatment.
In two Level I clinical studies, Kiva was shown to reduce the rate of adjacent level fractures and cement extravasation and restore the kyphotic angle. The implant mimics cancellous bone and...
IZI Medical Products received approval under the CE Mark for the unipedicular PEEK implant-based Kiva™ Vertebral Compression Fracture (VCF) Treatment.
In two Level I clinical studies, Kiva was shown to reduce the rate of adjacent level fractures and cement extravasation and restore the kyphotic angle. The implant mimics cancellous bone and provides structural support to prevent further collapse of the vertebral body. The unipedicular deployment method allows midline placement to support the axial vertebral body load.
IZI Medical acquired Benvenue Medical’s vertebral augmentation systems portfolio in 2018, including the Kiva VCF system, Kiva Pilot and Blazer Vertebral Augmentation System.
“We are excited to provide this novel implant technology to the European market and have made significant investments in our international distribution network to broaden our VCF offering,” said Greg Groenke, Chief Executive Officer, IZI Medical.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







