
 Copy to clipboard
Copy to clipboard 
Gyder Surgical was granted FDA 510(k) clearance to market the GYDER Hip System, the world’s first commercially available non-invasive (pin-less) and image-less solution for the accurate positioning of the acetabular cup during Anterior Hip Arthroplasty. The FDA’s clearance decision is the second significant regulatory milestone for GYDER Hip, which received Australia’s TGA regulatory approval previously. Surgical cases have already been successfully performed in Australia and India.
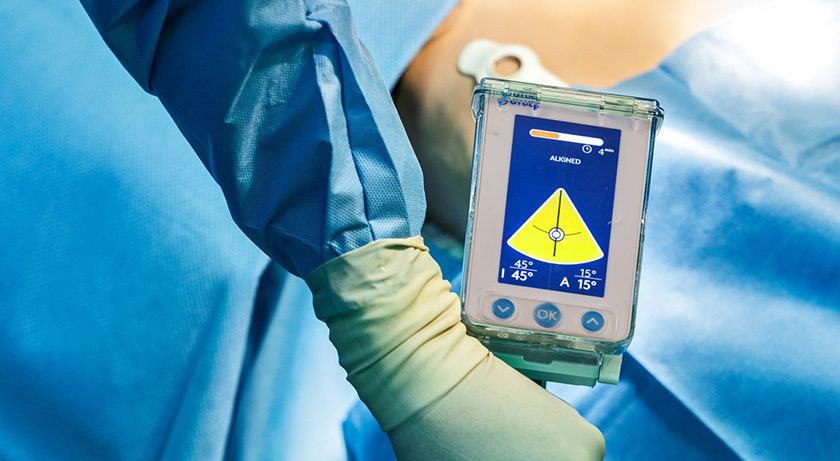
Unlike traditional hip navigation approaches, the GYDER Hip System’s patented technology does not use invasive metallic pins, nor does it rely on pre- or intra-operative imaging for anatomical landmark registration. One-minute calibration and quick registration support fast, easy-to-use computer-assisted navigation that integrates into existing surgical workflows.
“The FDA 510(k) clearance of the GYDER Hip System marks a significant milestone in bringing this innovation to surgeons in the world’s largest hip replacement market. The GYDER Hip System is well suited for use in the fast-growing Ambulatory Surgery Center (outpatient) segment, with its small footprint, low training burden, speed, and efficiency,” said Sujit Dike, CEO, Gyder Surgical.
Source: Gyder Surgical
Gyder Surgical was granted FDA 510(k) clearance to market the GYDER Hip System, the world’s first commercially available non-invasive (pin-less) and image-less solution for the accurate positioning of the acetabular cup during Anterior Hip Arthroplasty. The FDA’s clearance decision is the second significant regulatory milestone for GYDER Hip,...
Gyder Surgical was granted FDA 510(k) clearance to market the GYDER Hip System, the world’s first commercially available non-invasive (pin-less) and image-less solution for the accurate positioning of the acetabular cup during Anterior Hip Arthroplasty. The FDA’s clearance decision is the second significant regulatory milestone for GYDER Hip, which received Australia’s TGA regulatory approval previously. Surgical cases have already been successfully performed in Australia and India.
Unlike traditional hip navigation approaches, the GYDER Hip System’s patented technology does not use invasive metallic pins, nor does it rely on pre- or intra-operative imaging for anatomical landmark registration. One-minute calibration and quick registration support fast, easy-to-use computer-assisted navigation that integrates into existing surgical workflows.
“The FDA 510(k) clearance of the GYDER Hip System marks a significant milestone in bringing this innovation to surgeons in the world’s largest hip replacement market. The GYDER Hip System is well suited for use in the fast-growing Ambulatory Surgery Center (outpatient) segment, with its small footprint, low training burden, speed, and efficiency,” said Sujit Dike, CEO, Gyder Surgical.
Source: Gyder Surgical

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.