
 Copy to clipboard
Copy to clipboard 
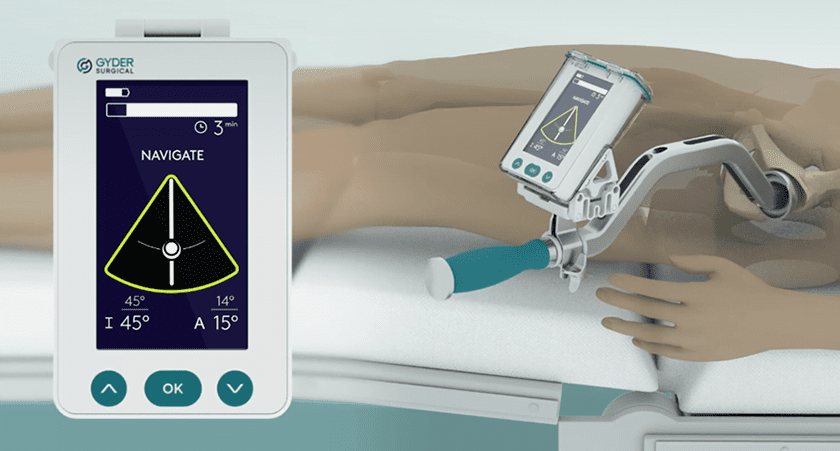
Gyder Surgical announced that the Therapeutic Goods Administration (TGA) of Australia granted regulatory approval for the GYDER Hip Navigation System, a non-invasive (Pin-less) and Image-less smart navigation system for total hip replacement surgery using the anterior approach.
GYDER Hip Navigation is a computer-controlled, non-invasive surgical tool to aid navigation during total hip replacement via the anterior approach. It is designed to provide real-time guidance to assist the surgeon in positioning the acetabular cup in the intended orientation during primary or revision hip replacement.
The GYDER system does not use invasive metallic pins utilized by most navigation products in the market. It also does not rely on pre-operative or inter-operative imaging such as computer tomography or x-ray technology. Calibration takes one minute, and registration is quick. It has a small footprint, seamlessly integrates into existing workflow, and is an open platform that provides surgeons flexibility to use their preferred implants. Gyder Surgical plans to commercialize the GYDER Hip Navigation System later this year.
“We are excited to receive the regulatory approval from Australia’s TGA for the GYDER Hip Navigation System. It’s a major milestone and an important step forward in our journey to bring our patented Pin-less and Image-less technology to orthopaedic surgeons in Australia and for improving patient care,” said Sujit Dike, CEO of Gyder Surgical. “We believe that there is a strong appetite for fast, easy-to-use and cost-effective enabling technologies that improve surgical accuracy during orthopaedic surgery.”
Source: Gyder Surgical
Gyder Surgical announced that the Therapeutic Goods Administration (TGA) of Australia granted regulatory approval for the GYDER Hip Navigation System, a non-invasive (Pin-less) and Image-less smart navigation system for total hip replacement surgery using the anterior approach.
GYDER Hip Navigation is a computer-controlled, non-invasive...
Gyder Surgical announced that the Therapeutic Goods Administration (TGA) of Australia granted regulatory approval for the GYDER Hip Navigation System, a non-invasive (Pin-less) and Image-less smart navigation system for total hip replacement surgery using the anterior approach.
GYDER Hip Navigation is a computer-controlled, non-invasive surgical tool to aid navigation during total hip replacement via the anterior approach. It is designed to provide real-time guidance to assist the surgeon in positioning the acetabular cup in the intended orientation during primary or revision hip replacement.
The GYDER system does not use invasive metallic pins utilized by most navigation products in the market. It also does not rely on pre-operative or inter-operative imaging such as computer tomography or x-ray technology. Calibration takes one minute, and registration is quick. It has a small footprint, seamlessly integrates into existing workflow, and is an open platform that provides surgeons flexibility to use their preferred implants. Gyder Surgical plans to commercialize the GYDER Hip Navigation System later this year.
“We are excited to receive the regulatory approval from Australia’s TGA for the GYDER Hip Navigation System. It’s a major milestone and an important step forward in our journey to bring our patented Pin-less and Image-less technology to orthopaedic surgeons in Australia and for improving patient care,” said Sujit Dike, CEO of Gyder Surgical. “We believe that there is a strong appetite for fast, easy-to-use and cost-effective enabling technologies that improve surgical accuracy during orthopaedic surgery.”
Source: Gyder Surgical

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







