
 Copy to clipboard
Copy to clipboard 
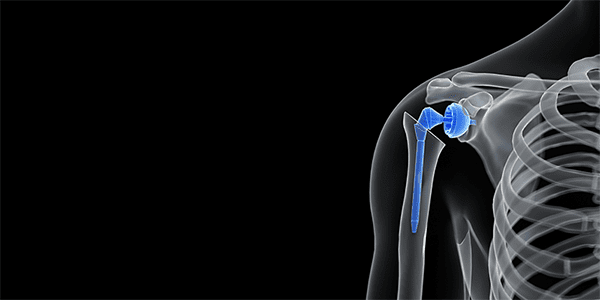
Genesis Software Innovations (GSI) received FDA 510(k) clearance to market PreView™, 3D shoulder replacement planning software based on CT imaging studies. PreView shows a representation of the patient’s shoulder anatomy as a 3D model, allowing for digital placement of the implant and optimization of implant size, location and orientation. This clearance is the first orthopedic solution under FDA’s new QIH classification for software solutions based on Artificial Intelligence.
PreView software will be available in the coming month.
GSI’s platform is designed to improve efficiencies, decrease costs and improve patient outcomes and satisfaction. Its functional visualization brings clarity to the procedure before entering the O.R., and reduces total O.R. time.
Other tools in GSI’s platform include:
- GSI Recon Inventory™ to address inventory and sterilization for hospitals and surgery centers
- GSI Insight™ for data collection and patient satisfaction
- GSI Touch™ patient optimization software to assist in surgery preparation and recovery
“We are incredibly excited about the FDA 510(k) clearance for PreView™. We anticipate this to be the first of many announcements as our platform expands to include additional surgical applications throughout the anatomy,” said Matt Miller, Senior Director, Technology Development for GSI.
Genesis Software Innovations (GSI) received FDA 510(k) clearance to market PreView™, 3D shoulder replacement planning software based on CT imaging studies. PreView shows a representation of the patient's shoulder anatomy as a 3D model, allowing for digital placement of the implant and optimization of implant size, location and orientation....
Genesis Software Innovations (GSI) received FDA 510(k) clearance to market PreView™, 3D shoulder replacement planning software based on CT imaging studies. PreView shows a representation of the patient’s shoulder anatomy as a 3D model, allowing for digital placement of the implant and optimization of implant size, location and orientation. This clearance is the first orthopedic solution under FDA’s new QIH classification for software solutions based on Artificial Intelligence.
PreView software will be available in the coming month.
GSI’s platform is designed to improve efficiencies, decrease costs and improve patient outcomes and satisfaction. Its functional visualization brings clarity to the procedure before entering the O.R., and reduces total O.R. time.
Other tools in GSI’s platform include:
- GSI Recon Inventory™ to address inventory and sterilization for hospitals and surgery centers
- GSI Insight™ for data collection and patient satisfaction
- GSI Touch™ patient optimization software to assist in surgery preparation and recovery
“We are incredibly excited about the FDA 510(k) clearance for PreView™. We anticipate this to be the first of many announcements as our platform expands to include additional surgical applications throughout the anatomy,” said Matt Miller, Senior Director, Technology Development for GSI.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







