
 Copy to clipboard
Copy to clipboard 
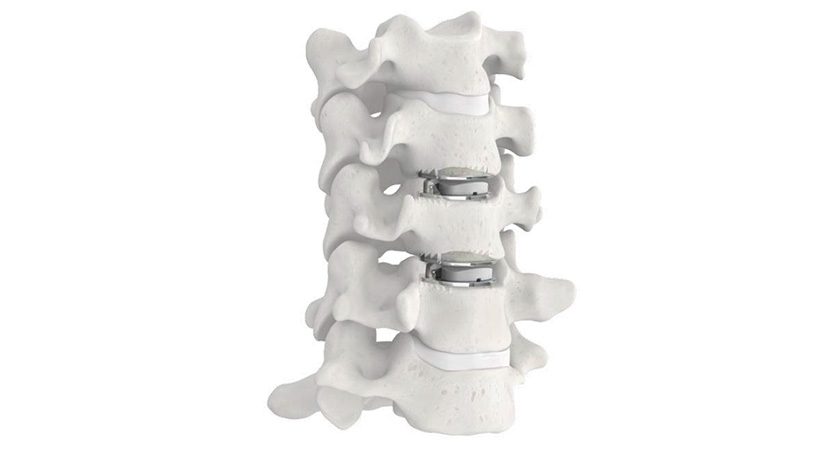
ZimVie announced that the first patient in the United States has received the 4.5mm Mobi-C Cervical Disc. Introduced in France in 2004, Mobi-C became the first cervical disc approved for one and two levels by the FDA in 2013. The smaller 4.5mm height Mobi-C implants, available in seven footprints to match patient anatomy, were approved by FDA in August of this year.
Mobi-C is the first cervical disc prosthesis approved by FDA for reconstruction of a cervical disc at both one and two levels (C3-C7). Mobi-C is a cobalt chromium alloy and polyethylene mobile-bearing prosthesis that is inserted in a single step, without requiring bone chiseling or other vertebral anchorage such as screws or keels. The device is implanted using an anterior approach.
Source: ZimVie
ZimVie announced that the first patient in the United States has received the 4.5mm Mobi-C Cervical Disc. Introduced in France in 2004, Mobi-C became the first cervical disc approved for one and two levels by the FDA in 2013. The smaller 4.5mm height Mobi-C implants, available in seven footprints to match patient anatomy, were approved by FDA...
ZimVie announced that the first patient in the United States has received the 4.5mm Mobi-C Cervical Disc. Introduced in France in 2004, Mobi-C became the first cervical disc approved for one and two levels by the FDA in 2013. The smaller 4.5mm height Mobi-C implants, available in seven footprints to match patient anatomy, were approved by FDA in August of this year.
Mobi-C is the first cervical disc prosthesis approved by FDA for reconstruction of a cervical disc at both one and two levels (C3-C7). Mobi-C is a cobalt chromium alloy and polyethylene mobile-bearing prosthesis that is inserted in a single step, without requiring bone chiseling or other vertebral anchorage such as screws or keels. The device is implanted using an anterior approach.
Source: ZimVie

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







