
 Copy to clipboard
Copy to clipboard 
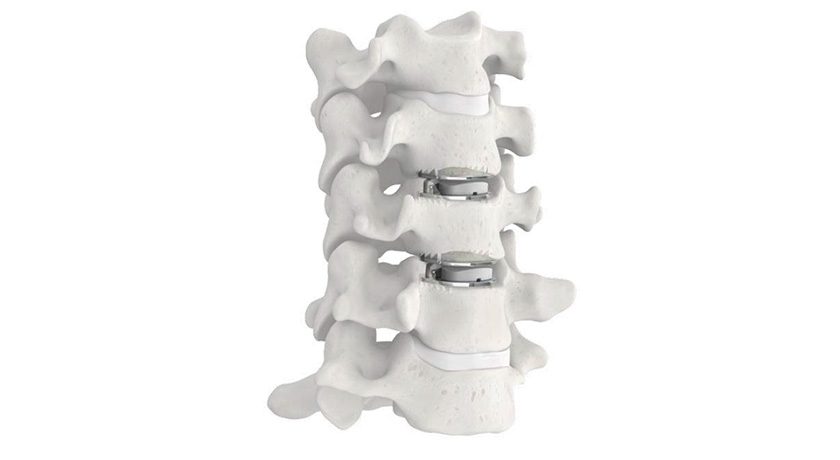
ZimVie gained FDA premarket approval for a smaller height of the Mobi-C Cervical Disc in seven footprints that will address the anatomical needs of the U.S. patient population.
Mobi-C is the first cervical disc prosthesis approved by FDA for reconstruction of a cervical disc at both one and two levels (C3-C7). Mobi-C is a cobalt chromium alloy and polyethylene mobile-bearing prosthesis that is inserted in a single step, without requiring bone chiseling or other vertebral anchorage such as screws or keels.
Physicians have used Mobi-C for cervical disc replacement at one level or two contiguous levels to treat patients in France since 2004 and in the U.S. since 2013 when it became the first cervical disc approved for one and two levels by FDA.
“The approval of the 4.5mm Mobi-C is a win for our surgeons and their patients, as well as a validation of thoughtful strategy by our global Regulatory Affairs team who utilized real-world clinical evidence gained from EU studies to show long-term safety and efficacy and secure the FDA approval for the smaller disc,” said Rebecca Whitney, Global President of ZimVie Spine. “We are pleased to provide surgeons the largest range of footprint and height options in the market to bring motion preservation to their patients. We will commercialize the product in the U.S. this fall.”
Source: ZimVie
ZimVie gained FDA premarket approval for a smaller height of the Mobi-C Cervical Disc in seven footprints that will address the anatomical needs of the U.S. patient population.
Mobi-C is the first cervical disc prosthesis approved by FDA for reconstruction of a cervical disc at both one and two levels (C3-C7). Mobi-C is a cobalt chromium alloy...
ZimVie gained FDA premarket approval for a smaller height of the Mobi-C Cervical Disc in seven footprints that will address the anatomical needs of the U.S. patient population.
Mobi-C is the first cervical disc prosthesis approved by FDA for reconstruction of a cervical disc at both one and two levels (C3-C7). Mobi-C is a cobalt chromium alloy and polyethylene mobile-bearing prosthesis that is inserted in a single step, without requiring bone chiseling or other vertebral anchorage such as screws or keels.
Physicians have used Mobi-C for cervical disc replacement at one level or two contiguous levels to treat patients in France since 2004 and in the U.S. since 2013 when it became the first cervical disc approved for one and two levels by FDA.
“The approval of the 4.5mm Mobi-C is a win for our surgeons and their patients, as well as a validation of thoughtful strategy by our global Regulatory Affairs team who utilized real-world clinical evidence gained from EU studies to show long-term safety and efficacy and secure the FDA approval for the smaller disc,” said Rebecca Whitney, Global President of ZimVie Spine. “We are pleased to provide surgeons the largest range of footprint and height options in the market to bring motion preservation to their patients. We will commercialize the product in the U.S. this fall.”
Source: ZimVie

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







