
 Copy to clipboard
Copy to clipboard 
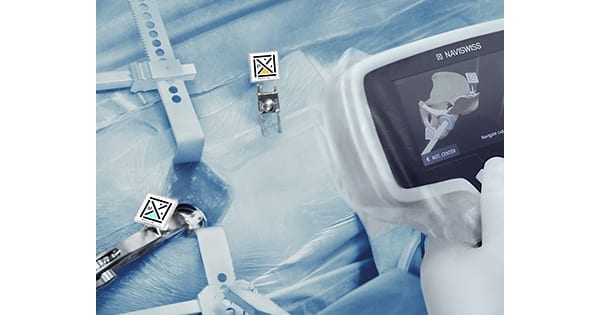
Naviswiss U.S. announced the first successful U.S. surgery with its hand-held hip navigation system following FDA 510(k) clearance in 2Q20.
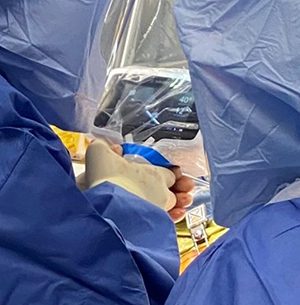
Naviswiss navigation in hip replacement surgery
Indicated for primary and revision hip replacement procedures, the system uses proprietary tracking technology with 95% smaller trackers to provide real-time measurements down to the degree for anteversion, inclination, leg length and offset. The open platform works with all major hip implants and approaches.
Naviswiss plans to expand usage in New York and throughout the U.S. in 2020 with a fee-per-use model rather than requiring a hospital or ASC to purchase the system. It is easily transported between O.R.s, supporting multiple procedures. The system also documents final implantation parameters that allow surgeons and patients to review the final surgical results.
Last month, the company announced completion of 1,000 navigated total hip replacement cases worldwide.
Naviswiss U.S. announced the first successful U.S. surgery with its hand-held hip navigation system following FDA 510(k) clearance in 2Q20.
Indicated for primary and revision hip replacement procedures, the system uses proprietary tracking technology with 95% smaller trackers to provide real-time measurements down to the degree for...
Naviswiss U.S. announced the first successful U.S. surgery with its hand-held hip navigation system following FDA 510(k) clearance in 2Q20.

Naviswiss navigation in hip replacement surgery
Indicated for primary and revision hip replacement procedures, the system uses proprietary tracking technology with 95% smaller trackers to provide real-time measurements down to the degree for anteversion, inclination, leg length and offset. The open platform works with all major hip implants and approaches.
Naviswiss plans to expand usage in New York and throughout the U.S. in 2020 with a fee-per-use model rather than requiring a hospital or ASC to purchase the system. It is easily transported between O.R.s, supporting multiple procedures. The system also documents final implantation parameters that allow surgeons and patients to review the final surgical results.
Last month, the company announced completion of 1,000 navigated total hip replacement cases worldwide.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







