
 Copy to clipboard
Copy to clipboard 
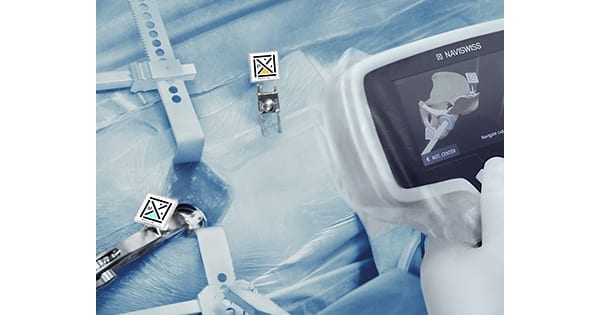
Naviswiss received FDA 510(k) clearance for their navigation technology for hip replacement surgery. The clearance addresses primary and revision hip replacement with a variety of surgical approaches. The system can be used with most implants on the market.
This builds upon the system’s previous availability in Europe, Australia and Asia.
Naviswiss has implemented a fee-per-use business model, providing technology for a low per-case cost and eliminating the need for large investments from its customers.
CEO Jan Stifter said, “We are extremely excited to provide our core products to the U.S. market. The Naviswiss system is unique in both form and function. Rather than using off-the-shelf tracking components like most other navigation systems, we developed our core technology to be smaller, lighter and more accurate. In doing so we reduced the cost of a computer assisted hip and empowered surgeons to have more information about the specific patient anatomy.”
Naviswiss received FDA 510(k) clearance for their navigation technology for hip replacement surgery. The clearance addresses primary and revision hip replacement with a variety of surgical approaches. The system can be used with most implants on the market.
This builds upon the system's previous availability in Europe, Australia and...
Naviswiss received FDA 510(k) clearance for their navigation technology for hip replacement surgery. The clearance addresses primary and revision hip replacement with a variety of surgical approaches. The system can be used with most implants on the market.
This builds upon the system’s previous availability in Europe, Australia and Asia.
Naviswiss has implemented a fee-per-use business model, providing technology for a low per-case cost and eliminating the need for large investments from its customers.
CEO Jan Stifter said, “We are extremely excited to provide our core products to the U.S. market. The Naviswiss system is unique in both form and function. Rather than using off-the-shelf tracking components like most other navigation systems, we developed our core technology to be smaller, lighter and more accurate. In doing so we reduced the cost of a computer assisted hip and empowered surgeons to have more information about the specific patient anatomy.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







