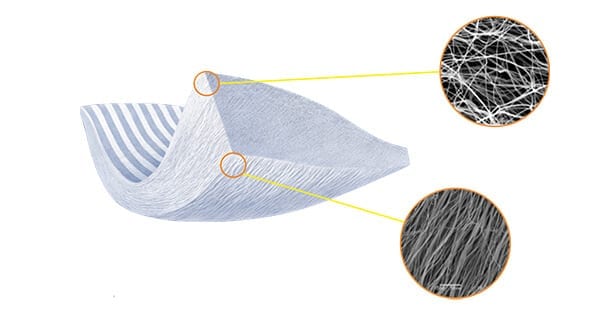






Embody announced the expansion and clinical use cases of TAPESTRY® Biointegrative Implant in the U.S. TAPESTRY is reportedly the first collagen-based implant with a bioengineered micro-architecture and biostimulative type I collagen chemistry, specifically designed for the management and protection of tendon injuries.
The combination of collagen chemistry and structural integrity provides clinical utility and sizing across a broad range of tendon applications including, but not limited to, foot/ankle, knee and shoulder procedures.
The device received FDA 510(k) clearance in 4Q20 for tendon and ligament repair.
“Following the initial surgical procedures with TAPESTRY, we are seeing continued use and expanded interest across a wide range of demanding tendon applications including subscapularis, patellar, peroneal and Achilles tendons where formative products have lacked the combination of handling, structural integrity, and a biostimulative chemistry,” said Jeff Conroy, CEO of Embody. “We continue to expand our range of sizes and shapes to rapidly accommodate every surgeon’s need.”
Embody announced the expansion and clinical use cases of TAPESTRY® Biointegrative Implant in the U.S. TAPESTRY is reportedly the first collagen-based implant with a bioengineered micro-architecture and biostimulative type I collagen chemistry, specifically designed for the management and protection of tendon injuries.
The combination of...
Embody announced the expansion and clinical use cases of TAPESTRY® Biointegrative Implant in the U.S. TAPESTRY is reportedly the first collagen-based implant with a bioengineered micro-architecture and biostimulative type I collagen chemistry, specifically designed for the management and protection of tendon injuries.
The combination of collagen chemistry and structural integrity provides clinical utility and sizing across a broad range of tendon applications including, but not limited to, foot/ankle, knee and shoulder procedures.
The device received FDA 510(k) clearance in 4Q20 for tendon and ligament repair.
“Following the initial surgical procedures with TAPESTRY, we are seeing continued use and expanded interest across a wide range of demanding tendon applications including subscapularis, patellar, peroneal and Achilles tendons where formative products have lacked the combination of handling, structural integrity, and a biostimulative chemistry,” said Jeff Conroy, CEO of Embody. “We continue to expand our range of sizes and shapes to rapidly accommodate every surgeon’s need.”


You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







